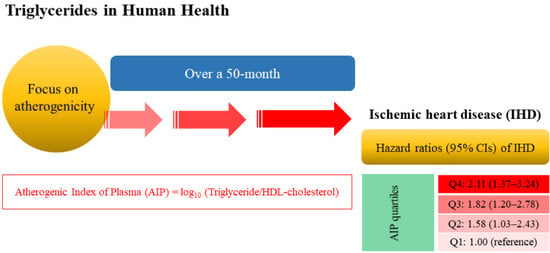
Predictive Value of the Atherogenic Index of Plasma (AIP) for the Risk of Incident Ischemic Heart Disease among Non-Diabetic Koreans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes
2.3. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ab Khan, M.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; Alkatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global epidemiology of ischemic heart disease: Results from the global burden of disease study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-H.; Cho, S.M.J.; Lee, H.; Baek, J.; Bae, J.-H.; Chung, W.-J.; Kim, H.C. Korea heart disease fact sheet 2020: Analysis of nationwide data. Korean Circ. J. 2021, 51, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Castelli, W. Epidemiology of coronary heart disease: The framingham study. Am. J. Med. 1984, 76, 4–12. [Google Scholar] [CrossRef]
- Stamler, J.; Wentworth, D.; Neaton, J.D. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the multiple risk factor intervention trial (MRFIT). JAMA 1986, 256, 2823–2828. [Google Scholar] [CrossRef] [PubMed]
- Lipid Research Clinics Program. The lipid research clinics coronary primary prevention trail results. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984, 251, 365–374. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Z.; Zhu, L.; Ouyang, X.; Yang, Y.; He, W.; Feng, Y.; Yi, F.; Song, Y. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiol. Pol. 2015, 73, 931–938. [Google Scholar] [CrossRef]
- Hamsten, A.; Walldius, G.; Dahlén, G.; Johansson, B.; De Faire, U. Serum lipoproteins and apolipoproteins in young male survivors of myocardial infarction. Atherosclerosis 1986, 59, 223–235. [Google Scholar] [CrossRef]
- Brunner, D.; Altman, S.; Loebl, K.; Schwartz, S.; Levin, S. Serum cholesterol and triglycerides in patients suffering from ischemic heart disease and in healthy subjects. Atherosclerosis 1977, 28, 197–204. [Google Scholar] [CrossRef]
- Tverdal, A.; Foss, O.P.; Leren, P.; Holme, I.; Lund-Larsen, P.G.; Bjartveit, K. Serum triglycerides as an independent risk factor for death from coronary heart disease in mtddle-aged Norwegian men. Am. J. Epidemiol. 1989, 129, 458–465. [Google Scholar] [CrossRef]
- Petersson, B.; Trell, E.; Hood, B. Premature death and associated risk factors in urban middle-aged men. Am. J. Med. 1984, 77, 418–426. [Google Scholar] [CrossRef]
- Jeppesen, J.Ø.; Hein, H.O.; Suadicani, P.; Gyntelberg, F. Triglyceride concentration and ischemic heart disease: An eight-year follow-up in the copenhagen male study. Circulation 1998, 97, 1029–1036. [Google Scholar] [CrossRef] [Green Version]
- Austin, M.A. Plasma triglyceride and coronary heart disease. Arter. Thromb. J. Vasc. Biol. 1991, 11, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, D.; Witztum, J.L. Lipoproteins and atherogenesis. Current concepts. JAMA 1990, 264, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Hulley, S.B.; Rosenman, R.H.; Bawol, R.D.; Brand, R.J. Epidemiology as a guide to clinical decisions: The association between triglyceride and coronary heart disease. N. Engl. J. Med. 1980, 302, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease: The framingham study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef]
- Miller, G.; Miller, N. Plasma-high-density-lipoprotein concentration and development of ischæmic heart-disease. Lancet 1975, 305, 16–19. [Google Scholar] [CrossRef]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of coronary heart disease and lipoprotein cholesterol levels. The framingham study. JAMA 1986, 256, 2835–2838. [Google Scholar] [CrossRef]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic index of plasma (AIP): A marker of cardiovascular disease. Med. J. Islam. Repub. Iran 2015, 29, 240. [Google Scholar]
- Scicali, R.; Giral, P.; D’Erasmo, L.; Cluzel, P.; Redheuil, A.; Di Pino, A.; Rabuazzo, A.M.; Piro, S.; Arca, M.; Béliard, S. High tg to hdl ratio plays a significant role on atherosclerosis extension in prediabetes and newly diagnosed type 2 diabetes subjects. Diabetes Metab. Res. Rev. 2021, 37, e3367. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Baral, B.; Raut, M.; Khanal, M. Atherogenic index of plasma for prediction of future cardiovascular disease in prediabetes and diabetes population. Atherosclerosis 2016, 252, e120. [Google Scholar] [CrossRef]
- Wakabayashi, I. Associations of blood lipid-related indices with blood pressure and pulse pressure in middle-aged men. Metab. Syndr. Relat. Disord. 2015, 13, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.M.; Wang, J.; Morris, A.D. Comparison of cardiovascular risk between patients with type 2 diabetes and those who had had a myocardial infarction: Cross sectional and cohort studies. BMJ 2002, 324, 939. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.D.; Folsom, A.R.; Pankow, J.S.; Brancati, F.L. Cardiovascular events in diabetic and nondiabetic adults with or without history of myocardial infarction. Circulation 2004, 109, 855–860. [Google Scholar] [CrossRef] [Green Version]
- Park, B.; Lee, Y.-J.; Lee, H.S.; Jung, D.-H. The triglyceride-glucose index predicts ischemic heart disease risk in Koreans: A prospective study using National Health Insurance Service data. Cardiovasc. Diabetol. 2020, 19, 210. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, B.-Y.; Kang, E.S.; Noh, J.; Kim, S.-K.; Park, S.-O.; Hur, K.Y.; Chon, S.; Moon, M.K. Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab. J. 2019, 43, 398–406. [Google Scholar] [CrossRef]
- Carretero, O.A.; Oparil, S. Essential hypertension: Part I: Definition and etiology. Circulation 2000, 101, 329–335. [Google Scholar] [CrossRef]
- Genuth, S.; Alberti, K.G.; Bennett, P.; Buse, J.; Defronzo, R.; Kahn, R.; Kitzmiller, J.; Knowler, W.C.; Lebovitz, H.; Lernmark, A.; et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003, 26, 3160–3167. [Google Scholar]
- Einhorn, D. American college of endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003, 9, 5–21. [Google Scholar] [CrossRef]
- Dobiášová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FERHDL). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Dobiásová, M.; Frohlich, J. The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate: Changes during lipanor therapy. Vnitrni Lekarstvi 2000, 46, 152–156. [Google Scholar] [PubMed]
- Dobiásová, M. AIP—atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitrni Lekarstvi 2006, 52, 64–71. [Google Scholar] [PubMed]
- Bittner, V.; Johnson, B.D.; Zineh, I.; Rogers, W.J.; Vido, D.; Marroquin, O.C.; Bairey-Merz, C.N.; Sopko, G. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: A report from the women’s ischemia syndrome evaluation (wise). Am. Heart J. 2009, 157, 548–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, K.; Zhao, J.; Huang, H.; Zhang, Q.; Chen, X.; Zeng, Z.; Zhang, L.; Chen, Y. The association between triglyceride/high-density lipoprotein cholesterol ratio and all-cause mortality in acute coronary syndrome after coronary revascularization. PLoS ONE 2015, 10, e0123521. [Google Scholar] [CrossRef]
- Wu, T.-T.; Gao, Y.; Zheng, Y.-Y.; Ma, Y.-T.; Xie, X. Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018, 17, 197. [Google Scholar] [CrossRef]
- Dobiášová, M. Atherogenic index of plasma [log (triglycerides/HDL-cholesterol)]: Theoretical and practical implications. Clin. Chem. 2004, 50, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Onat, A.; Can, G.; Kaya, H.; Hergenç, G. “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein−cholesterol) predicts high blood pressure, diabetes, and vascular events. J. Clin. Lipidol. 2010, 4, 89–98. [Google Scholar] [CrossRef]
- Zhu, X.-W.; Deng, F.-Y.; Lei, S.-F. Meta-analysis of atherogenic index of plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim. Care Diabetes 2015, 9, 60–67. [Google Scholar] [CrossRef]
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Grundy, S.M. Small LDL, atherogenic dyslipidemia, and the metabolic syndrome. Circulation 1997, 95, 1–4. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Syndrome x: What’s old, what’s new, what’s etiologic? J. Clin. Investig. 1993, 92, 3. [Google Scholar] [CrossRef]
- Lemieux, I.; Després, J.-P. Metabolic syndrome: Past, present and future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Wilson, P.W.F.; Fox, C.S.; Vasan, R.S.; Nathan, D.M.; Sullivan, L.; D’Agostino, R.B. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J. Clin. Endocrinol. Metab. 2006, 91, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Targher, G.; Formentini, G.; Calcaterra, F.; Lombardi, S.; Marini, F.; Zenari, L.; Saggiani, F.; Poli, M.; Perbellini, S.; et al. The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects. Prospective data from the verona diabetes complications study. Diabet. Med. 2004, 21, 52–58. [Google Scholar] [CrossRef]
- Ninomiya, T.; Kubo, M.; Doi, Y.; Yonemoto, K.; Tanizaki, Y.; Rahman, M.; Arima, H.; Tsuryuya, K.; Iida, M.; Kiyohara, Y. Impact of metabolic syndrome on the development of cardiovascular disease in a general Japanese population: The hisayama study. Stroke 2007, 38, 2063–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizargar, J.; Bai, C.-H.; Hsieh, N.-C.; Wu, S.-F.V. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc. Diabetol. 2020, 19, 8. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-C.; He, G.-D.; Lo, K.; Huang, Y.-Q.; Feng, Y.-Q. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front. Cardiovasc. Med. 2021, 7, 628109. [Google Scholar] [CrossRef]
- Si, Y.; Fan, W.; Han, C.; Liu, J.; Sun, L. Atherogenic index of plasma, triglyceride-glucose index and monocyte-to-lymphocyte ratio for predicting subclinical coronary artery disease. Am. J. Med. Sci. 2021. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American heart association’s strategic impact goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, S.; Rangarajan, S.; Teo, K.; Islam, S.; Li, W.; Liu, L.; Bo, J.; Lou, Q.; Lu, F.; Liu, T.; et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N. Engl. J. Med. 2014, 371, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.G.; Blaha, M.J.; Krumholz, H.M.; Budoff, M.J.; Blankstein, R.; Sibley, C.T.; Agatston, A.; Blumenthal, R.S.; Nasir, K. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: The multi-ethnic study of atherosclerosis. Eur. Heart J. 2014, 35, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Merz, C.N.B.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study: Part I: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J. Am. Coll. Cardiol. 2006, 47, S4–S20. [Google Scholar] [CrossRef] [Green Version]
- Wake, R.; Takeuchi, M.; Yoshikawa, J.; Yoshiyama, M. Effects of gender on prognosis of patients with known or suspected coronary artery disease undergoing contrast-enhanced dobutamine stress echocardiography. Circ. J. 2007, 71, 1060–1066. [Google Scholar] [CrossRef] [Green Version]
- Kwolek, C.J.; Clagett, G.P. Changing demographics in patients with vascular disease. J. Vasc. Surg. 2009, 49, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khakurel, G.; Kayastha, R.; Chalise, S.; Karki, P.K. Atherogenic index of plasma in postmenopausal women. J. Nepal Health Res. Counc. 2018, 16, 175–177. [Google Scholar] [CrossRef]
- Anagnostis, P.; Stevenson, J.C.; Crook, D.; Johnston, D.; Godsland, I.F. Effects of gender, age and menopausal status on serum apolipoprotein concentrations. Clin. Endocrinol. 2016, 85, 733–740. [Google Scholar] [CrossRef] [PubMed]


| AIP Quartiles | ||||||
|---|---|---|---|---|---|---|
| Q1 n = 4529 | Q2 n = 4358 | Q3 n = 4582 | Q4 n = 4475 | p Value 1 | Post Hoc 2 | |
| AIP | ≤−0.27 | −0.26 to −0.08 | −0.07 to 0.14 | ≥ 0.15 | ||
| Age (years) | 42.3 ± 10.5 | 44.6 ± 10.6 | 46.1 ± 10.4 | 45.9 ± 10.0 | <0.001 | a,b,c,d,e |
| Male sex (%) | 24.4 | 42.4 | 60.6 | 76.5 | <0.001 | - |
| Body mass index (kg/m2) | 21.4 ± 2.5 | 22.6 ± 2.7 | 23.9 ± 2.8 | 25.1 ± 2.8 | <0.001 | a,b,c,d,e,f |
| Systolic blood pressure (mmHg) | 115.4 ± 14.3 | 119.8 ± 15.1 | 123.8 ± 14.9 | 127.8 ± 14.7 | <0.001 | a,b,c,d,e,f |
| Diastolic blood pressure (mmHg) | 71.7 ± 9.3 | 74.6 ± 9.8 | 77.3 ± 9.8 | 80.1 ± 9.6 | <0.001 | a,b,c,d,e,f |
| Mean arterial pressure (mmHg) | 86.2 ± 10.6 | 89.7 ± 11.2 | 92.8 ± 11.1 | 96.0 ± 10.9 | <0.001 | a,b,c,d,e,f |
| Fasting plasma glucose (mmol/L) | 4.9 ± 0.5 | 5.0 ± 0.5 | 5.1 ± 0.5 | 5.2 ± 0.6 | <0.001 | a,b,c,d,e,f |
| Total cholesterol (mmol/L) | 4.7 ± 0.8 | 4.8 ± 0.8 | 5.0 ± 0.9 | 5.2 ± 0.9 | <0.001 | a,b,c,d,e,f |
| Triglyceride (mmol/L) | 0.7 ± 0.1 | 1.0 ± 0.2 | 1.4 ± 0.3 | 2.5 ± 1.2 | <0.001 | a,b,c,d,e,f |
| HDL-cholesterol (mmol/L) | 1.7 ± 0.3 | 1.5 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.2 | <0.001 | a,b,c,d,e,f |
| C-reactive protein (mg/L) | 0.9 ± 2.6 | 1.3 ± 3.4 | 1.6 ± 5.1 | 1.7 ± 3.5 | <0.001 | a,b,c,d,e |
| Current smoker (%) | 11.0 | 18.7 | 28.2 | 41.3 | <0.001 | - |
| Alcohol drinking (%) 3 | 36.1 | 41.3 | 45.7 | 52.5 | <0.001 | - |
| Regular exercise (%) 4 | 32.1 | 32.7 | 30.1 | 26.1 | <0.001 | - |
| Hypertension (%) | 10.0 | 15.4 | 22.8 | 30.5 | <0.001 | - |
| Impaired fasting glucose (%) | 7.9 | 13.2 | 20.0 | 27.6 | <0.001 | |
| Metabolic syndrome (%) | 0.6 | 2.1 | 7.3 | 36.1 | <0.001 | - |
| New Cases of Ischemic Heart Disease (IHD), n | Mean Follow-Up, Year | Pearson-Years of Follow-Up | Incidence Rate/1000 Person-Years | |
|---|---|---|---|---|
| Q1 | 38 | 2.3 ± 1.1 | 10,550 | 3.6 |
| Q2 | 73 | 2.4 ± 1.1 | 10,331 | 7.1 |
| Q3 | 98 | 2.3 ± 1.1 | 10,752 | 9.1 |
| Q4 | 123 | 2.4 ± 1.1 | 10,700 | 11.5 |
| HRs | 95% CIs | p Value | |
|---|---|---|---|
| Age, years | 1.071 | 1.061–1.081 | <0.001 |
| Male sex, yes or no | 1.614 | 1.291–2.018 | <0.001 |
| Body mass index, kg/m2 | 1.100 | 1.065–1.137 | <0.001 |
| Systolic blood pressure, mmHg | 1.017 | 1.010–1.023 | <0.001 |
| Diastolic blood pressure, mmHg | 1.025 | 1.015–1.036 | <0.001 |
| Fasting plasma glucose, mg/dL | 1.034 | 1.024–1.045 | <0.001 |
| Triglyceride, mg/dL | 1.001 | 1.001–1.002 | <0.001 |
| HDL-cholesterol, mg/dL | 0.978 | 0.969–0.987 | <0.001 |
| C-reactive protein, mg/L | 1.012 | 0.993–1.031 | 0.218 |
| Current smoker, yes or no | 1.140 | 0.854–1.521 | 0.374 |
| Alcohol drinking, yes or no | 0.722 | 0.573–0.909 | 0.006 |
| Regular exercise, yes or no | 1.499 | 1.193–1.803 | <0.001 |
| Atherogenic Index of Plasma (AIP) quartiles, Q1 vs. Q4 | 3.210 | 2.231–4.628 | <0.001 |
| Q1 | Q2 | Q3 | Q4 | p for Trend | ||
|---|---|---|---|---|---|---|
| Model 1 | HR (95% CI) | 1.00 (reference) | 1.63 (1.10–2.42) | 1.85 (1.27–2.71) | 2.34 (1.61–3.40) | <0.001 |
| p value | 0.015 | 0.001 | <0.001 | |||
| Model 2 | HR (95% CI) | 1.00 (reference) | 1.59 (1.04–2.45) | 1.87 (1.23–2.84) | 2.21 (1.45–3.39) | 0.003 |
| p value | 0.033 | 0.003 | <0.001 | |||
| Model 3 | HR (95% CI) | 1.00 (reference) | 1.58 (1.03–2.43) | 1.82 (1.20–2.78) | 2.11 (1.37–3.24) | 0.007 |
| p value | 0.037 | 0.005 | <0.001 | |||
| Q1 | Q2 | Q3 | Q4 | p for Trend | ||
|---|---|---|---|---|---|---|
| Model 1 | HR (95% CI) | 1.00 (reference) | 1.54 (0.85–2.80) | 1.88 (1.07–3.30) | 2.28 (1.32–3.94) | 0.013 |
| p value | 0.158 | 0.027 | 0.003 | |||
| Model 2 | HR (95% CI) | 1.00 (reference) | 1.43 (0.78–2.62) | 1.71 (0.96–3.03) | 1.89 (1.06–3.37) | 0.147 |
| p value | 0.244 | 0.068 | 0.030 | |||
| Model 3 | HR (95% CI) | 1.00 (reference) | 1.43 (0.78–2.62) | 1.65 (0.93–2.94) | 1.79 (1.00–3.19) | 0.238 |
| p value | 0.245 | 0.087 | 0.049 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.J.; Yoon, J.; Lee, Y.-J.; Park, B.; Jung, D.-H. Predictive Value of the Atherogenic Index of Plasma (AIP) for the Risk of Incident Ischemic Heart Disease among Non-Diabetic Koreans. Nutrients 2021, 13, 3231. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093231
Kim JJ, Yoon J, Lee Y-J, Park B, Jung D-H. Predictive Value of the Atherogenic Index of Plasma (AIP) for the Risk of Incident Ischemic Heart Disease among Non-Diabetic Koreans. Nutrients. 2021; 13(9):3231. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093231
Chicago/Turabian StyleKim, Julie J., Jihyun Yoon, Yong-Jae Lee, Byoungjin Park, and Dong-Hyuk Jung. 2021. "Predictive Value of the Atherogenic Index of Plasma (AIP) for the Risk of Incident Ischemic Heart Disease among Non-Diabetic Koreans" Nutrients 13, no. 9: 3231. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13093231