Antioxidant and Antihyperglycemic Effects of Ephedra foeminea Aqueous Extract in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
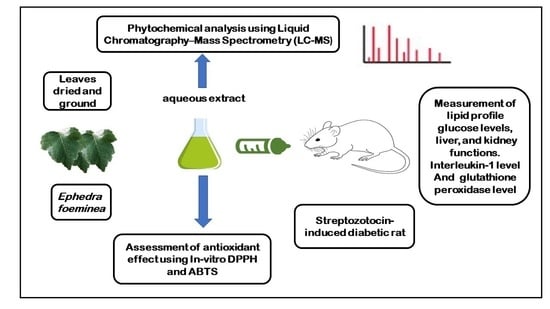
2. Material and Methods
2.1. Plant Materials
2.2. Aqueous Extract
2.3. Determination of Chemical Composition Using LC-MS
2.4. Antioxidant Activity
2.4.1. ABTS Free Radical Scavenging Assay
2.4.2. DPPH Radical Scavenging Activity
2.5. Antidiabetic Activity
2.5.1. Animals
2.5.2. Induction of Diabetes
2.5.3. Grouping of Animals and Experimental Procedure
- Group 1 (G1): Normal, nondiabetic rats were given citrate buffer on Day 1 (on the same day of STZ injection for the STZ-induced diabetic rats’ groups) and normal saline solution orally starting from Day 4.
- Group 2 (G2): Diabetic rats were given normal saline solution orally.
- Group 3 (G3): Diabetic rats were given metformin (100 mg/Kg) orally starting from Day 4.
- Group 4 (G4): Diabetic rats were given Ephedra foeminea extract (100 mg/Kg) orally starting from Day 4.
- Group 5 (G5): Diabetic rats were given Ephedra foeminea extract (100 mg/Kg) orally from Day 1 (on the same day of STZ injection) up to Day 4. The oral LD50 of Ephedra aqueous extract was reported to be in the range of 4000–8000 mg/kg [22].
2.6. Analysis of Blood Parameters
2.6.1. Interleukin 1beta Measurement
2.6.2. Glutathione Peroxidase Levels
2.7. Statistical Analysis
3. Results
3.1. Chemical Composition of Ephedra foeminea Aqueous Extract
3.2. Antioxidant Activity
3.3. Antidiabetic Activity
3.3.1. Blood Glucose Levels
3.3.2. Body Weight
3.4. Serum Lipid Profile (Cholesterol, Triglyceride, LDL, and HDL)
3.5. Serum Markers of Liver Function (ALT, AST, ALKP), Total Bilirubin, and Total Proteins
3.6. Evaluation of Kidney Function Test Biomarkers
3.7. Interleukin 1beta Level
3.8. Glutathione Peroxidase Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Al-Tawarah, N.M.; Qaralleh, H.; Khlaifat, A.M.; Nofal, M.N.; Alqaraleh, M.; Khleifat, K.M.; Al-limoun, M.O.; Al Shhab, M.A. Anticancer and Antibacterial Properties of Verthemia Iphionides Essential Oil/Silver Nanoparticles. Biomed. Pharmacol. J. 2020, 13, 1175–1184. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Al-Asoufi, A.; Khlaifat, A.; Tarawneh, A.; Alsharafa, K.; Al-Limoun, M.; Khleifat, K. Bacterial Quality of Urinary Tract Infections in Diabetic and Non Diabetics of the Population of Ma’an Province, Jordan. Pak. J. Biol. Sci. 2017, 20, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asano, T.; Shibasaki, Y.; Ohno, S.; Taira, H.; Lin, J.L.; Kasuga, M.; Kanazawa, Y.; Akanuma, Y.; Takaku, F.; Oka, Y. Rabbit brain glucose transporter responds to insulin when expressed in insulin-sensitive Chinese hamster ovary cells. J. Biol. Chem. 1989, 264, 3416–3420. [Google Scholar] [CrossRef]
- Khleifat, K.M.; Matar, S.A.; Jaafreh, M.; Qaralleh, H.; Al-limoun, M.O.; Alsharafa, K.Y. Essential Oil of Centaurea damascena Aerial Parts, Antibacterial and Synergistic Effect. J. Essent. Oil Bear. Plants 2019, 22, 356–367. [Google Scholar] [CrossRef]
- Khleifat, K.; Homady, M.H.; Tarawneh, K.A.; Shakhanbeh, J.J.E.J. Effect of Ferula hormonis extract on social aggression, fertility and some physiological parameters in prepubertal male mice. Endocr. J. 2001, 48, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The role of medicinal plants in the treatment of diabetes: A systematic review. Electron. Physician 2016, 8, 1832. [Google Scholar] [CrossRef] [Green Version]
- Andallu, B.; Varadacharyulu, N.C. Antioxidant role of mulberry (Morus indica L. cv. Anantha) leaves in streptozotocin-diabetic rats. Clin. Chim. Acta 2003, 338, 3–10. [Google Scholar] [CrossRef]
- Orhan, D.D.; Aslan, M.; Sendogdu, N.; Ergun, F.; Yesilada, E. Evaluation of the hypoglycemic effect and antioxidant activity of three Viscum album subspecies (European mistletoe) in streptozotocin-diabetic rats. J. Ethnopharmacol. 2005, 98, 95–102. [Google Scholar] [CrossRef]
- Khalil, M.; Khalifeh, H.; Saad, F.; Serale, N.; Salis, A.; Damonte, G.; Lupidi, G.; Daher, A.; Vergani, L. Protective effects of extracts from Ephedra foeminea Forssk fruits against oxidative injury in human endothelial cells. J. Ethnopharmacol. 2020, 260, 112976. [Google Scholar] [CrossRef]
- Zhang, B.-M.; Zhi-Bin, W.; Ping, X.; Qiu-Hong, W.; He, B.; Kuang, H.-X. Phytochemistry and pharmacology of genus Ephedra. Chin. J. Nat. Med. 2018, 16, 811–828. [Google Scholar] [CrossRef]
- Ziani, B.E.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C. Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starratt, A.N.; Caveney, S. Quinoline-2-carboxylic acids from Ephedra species. Phytochemistry 1996, 42, 1477–1478. [Google Scholar] [CrossRef]
- Davis, P.H. The New Phytologist. Flora Palaestina. JSTOR 1968, 67, 447–449. [Google Scholar]
- Jaiswal, D.; Rai, P.K.; Kumar, A.; Mehta, S.; Watal, G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 2009, 123, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Al Qaisi, Y.T.; Khleifat, K.M.; Oran, S.A.; Al Tarawneh, A.A.; Qaralleh, H.; Al-Qaisi, T.S.; Farah, H.S. Ruta graveolens, Peganum harmala, and Citrullus colocynthis methanolic extracts have in vitro protoscolocidal effects and act against bacteria isolated from echinococcal hydatid cyst fluid. Arch. Microbiol. 2022, 204, 228. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Jaafreh, M.; Khleifat, K.M.; Qaralleh, H.; Al-limoun, M.O. Antibacterial and Antioxidant Activities of Centeurea damascena Methanolic Extract. J. Basic Appl. Res. Biomed. 2019, 5, 55–63. [Google Scholar] [CrossRef]
- Hsu, B.; Coupar, I.M.; Ng, K. Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chem. 2006, 98, 317–328. [Google Scholar] [CrossRef]
- Alqaraleh, M.; Kasabri, V.; Al-Majali, I.; Aljaafreh, A.; Al-Othman, N.; Khleifat, K.; Al-Tawarah, N.M.; Qaralleh, H.; Khwaldeh, A.S.; Alalawi, S.; et al. Branched chain amino Acids as in vitro and in vivo Anti-Oxidation Compounds. Res. J. Pharm. Technol. 2021, 14, 3899–3904. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Al-Mustafa, A.H.; Tarawneh, K.; Khleifat, K.M.; Ridzwan, B.H.; Qaralleh, H.N. Protective role of Punica granatum L. peel extract against oxidative damage in experimental diabetic rats. Process Biochem. 2010, 45, 581–585. [Google Scholar] [CrossRef]
- Lee, M.R. The history of Ephedra (ma-huang). J. R. Coll. Physicians Edinb. 2011, 41, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Alsarayreh, A.Z.A.; Oran, S.A.; Shakhanbeh, J.M. Effect of Rhus coriaria L. methanolic fruit extract on wound healing in diabetic and non-diabetic rats. J. Cosmet. Dermatol. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.A. Renal syndromes in diabetes. Endocrinol. Metabol. Clin. 1996, 25, 293–324. [Google Scholar] [CrossRef]
- Lala, V.; Goyal, A.; Bansal, P.; Minter, D. Liver Function Tests; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. [Google Scholar]
- Gisbert, M.; Barcala, M.; Rosell, C.M.; Sineiro, J.; Moreira, R. Aqueous extracts characteristics obtained by ultrasound-assisted extraction from Ascophyllum nodosum seaweeds: Effect of operation conditions. J. Appl. Phycol. 2021, 33, 3297–3308. [Google Scholar] [CrossRef]
- Ibragic, S.; Sofić, E. Chemical composition of various Ephedra species. Bosn. J. Basic Med. Sci. 2015, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallassy, H.; Fayyad-Kazan, M.; Makki, R.; El-Makhour, Y.; Rammal, H.; Leger, D.Y.; Leger, D.Y.; Sol, V.; Fayyad-Kazan, H.; Liagre, B.; et al. Chemical composition and antioxidant, anti-inflammatory, and antiproliferative activities of Lebanese Ephedra Campylopoda plant. Med. Sci. Monit. Basic Res. 2017, 23, 313. [Google Scholar] [CrossRef] [Green Version]
- Merah, O.; Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Cerny, M.; Grivot, S.; Evon, P.; Hijazi, A. Biochemical composition of cumin seeds, and biorefining study. Biomolecules 2020, 10, 1054. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Yahia, Y.; Bettaieb, I.; Elfalleh, W.; Nagaz, K. Antioxidant activity and phenolic profile of a collection of medicinal plants from Tunisian arid and Saharan regions. Ind. Crops Prod. 2019, 138, 111427. [Google Scholar] [CrossRef]
- Mighri, H.; Akrout, A.; Bennour, N.; Eljeni, H.; Zammouri, T.; Neffati, M. LC/MS method development for the determination of the phenolic compounds of Tunisian Ephedra alata hydro-methanolic extract and its fractions and evaluation of their antioxidant activities. S. Afr. J. Bot. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Wang, D.; Dong, Y.; Wang, Q.; Wang, X.; Fan, W. Limonene, the compound in essential oil of nutmeg displayed antioxidant effect in sunflower oil during the deep-frying of Chinese Maye. Food Sci. Nutr. 2020, 8, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Wen, H.; Jiang, M.; Wu, F.; Tian, J.; Lu, X.; Xiao, J.; Liu, W. Effects of ferulic acid on growth performance, immunity and antioxidant status in genetically improved farmed tilapia (Oreochromis niloticus) fed oxidized fish oil. Aquac. Nutr. 2020, 26, 1431–1442. [Google Scholar] [CrossRef]
- Erendor, F.; Eksi, Y.E.; Sahin, E.O.; Balci, M.K.; Griffith, T.S.; Sanlioglu, S. Lentivirus mediated pancreatic beta-cell-specific insulin gene therapy for STZ-induced diabetes. Mol. Ther. 2021, 29, 149–161. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Song, R. Mechanism of metformin: A tale of two sites. Diabetes Care 2016, 39, 187–189. [Google Scholar] [CrossRef] [Green Version]
- Konno, C.; Mizuno, T.; Hikino, H. Isolation and hypoglycemic activity of ephedrans A, B, C, D and E, glycans of Ephedra distachya Herbs1. Planta Med. 1985, 51, 162–163. [Google Scholar] [CrossRef]
- Xiu, L.-M.; Miura, A.B.; Yamamoto, K.; Kobayashi, T.; Song, Q.-H.; Kitamura, H.; Cyong, J.C. Pancreatic islet regeneration by ephedrine in mice with streptozotocin-induced diabetes. Am. J. Chin. Med. 2001, 29, 493–500. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.; Lim, H.; Woo, S.; Shin, S.S.; Yoon, M. The herbal composition GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica inhibits visceral obesity and insulin resistance by upregulating visceral adipose genes involved in fatty acid oxidation. Pharm. Biol. 2015, 53, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Song, M.-K.; Um, J.-Y.; Jang, H.-J.; Lee, B.-C. Beneficial effect of dietary Ephedra sinica on obesity and glucose intolerance in high-fat diet-fed mice. Exp. Ther. Med. 2012, 3, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Meduru, H.; Wang, Y.-T.; Tsai, J.J.; Chen, Y.-C. Finding a potential dipeptidyl peptidase-4 (DPP-4) inhibitor for type-2 diabetes treatment based on molecular docking, pharmacophore generation, and molecular dynamics simulation. Int. J. Mol. Sci. 2016, 17, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeda-Montes, M.J.; Ardid-Ruiz, A.; Tomás-Hernández, S.; Gimeno, A.; Cereto-Massagué, A.; Beltrán-Debón, R.; Mulero, M.; Garcia-Vallvé, S.; Pujadas, G.; Valls, C. Ephedrine as a lead compound for the development of new DPP-IV inhibitors. Future Med. Chem. 2017, 9, 2129–2146. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Yang, J.-Y.; Lee, H.-S. Quinoline-2-carboxylic acid isolated from Ephedra pachyclada and its structural derivatives show inhibitory effects against α-glucosidase and α-amylase. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Hatting, M.; Tavares, C.D.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Ebbeling, C.B. The carbohydrate-insulin model of obesity: Beyond “calories in, calories out”. JAMA Int. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef]
- El-Ouady, F.; Lahrach, N.; Ajebli, M.; Haidani, A.E.; Eddouks, M. Antihyperglycemic effect of the aqueous extract of Foeniculum vulgare in normal and streptozotocin-induced diabetic rats. Cardiovasc. Haematol. Disord.-Drug Targets 2020, 20, 54–63. [Google Scholar] [CrossRef]
- Kalita, H.; Boruah, D.C.; Deori, M.; Hazarika, A.; Sarma, R.; Kumari, S.; Kandimalla, R.; Kotoky, J.; Devi, R. Antidiabetic and antilipidemic effect of Musa balbisiana root extract: A potent agent for glucose homeostasis in streptozotocin-induced diabetic rat. Front. Pharmacol. 2016, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Fontbonne, A.; Diouf, I.; Baccara-Dinet, M.; Eschwege, E.; Charles, M.-A. Effects of 1-year treatment with metformin on metabolic and cardiovascular risk factors in non-diabetic upper-body obese subjects with mild glucose anomalies: A post-hoc analysis of the BIGPRO1 trial. Diabetes Metabol. 2009, 35, 385–391. [Google Scholar] [CrossRef]
- Lee, A.; Morley, J.E. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes. Res. 1998, 6, 47–53. [Google Scholar] [CrossRef]
- Malin, S.K.; Nightingale, J.; Choi, S.E.; Chipkin, S.R.; Braun, B. Metformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose tolerant adults. Obesity 2013, 21, 93–100. [Google Scholar] [CrossRef]
- Seifarth, C.; Schehler, B.; Schneider, H. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp. Clin. Endocrinol. Diabetes 2013, 121, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, H.; Zhang, A.; Liang, Y.; Hao, J.; Zhang, X.; Lu, J. Effect of metformin on glycaemic control in patients with type 1 diabetes: A meta-analysis of randomized controlled trials. Diabetes Metabol. Res. Rev. 2018, 34, e2983. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; Deabes, M.; El-Askary, A.; El-Kott, A.F.; Albadrani, G.M.; Seif, A.; Wang, Z. Ephedra sinica mitigates hepatic oxidative stress and inflammation via suppressing the TLR4/MyD88/NF-κB pathway in fipronil-treated rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 62943–62958. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, J.; Yin, Q.; Zhang, Y.; Xu, H.; Shi, X.; Li, C.; Zhou, Y.; Zhou, C. Effect of extractions from Ephedra sinica Stapf on hyperlipidemia in mice. Exp. Ther. Med. 2015, 9, 619–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiss, M.; Souiy, Z.; Achour, L.; Hamden, K. Ephedra alata extracts exerts anti-obesity, anti-hyperglycemia, anti-antipyretic and analgesic effects. Nutr. Food Sci. 2021, 52, 119–128. [Google Scholar] [CrossRef]
- Wen, S.; Liao, T. Ephedrine causes liver toxicity in SD rats via oxidative stress and inflammatory responses. Hum. Exp. Toxicol. 2021, 40, 16–24. [Google Scholar] [CrossRef]
- Sioud, F.; Ben Toumia, I.; Lahmer, A.; Khlifi, R.; Dhaouefi, Z.; Maatouk, M.; Ghedira, K.; Chekir-Ghedira, L. Methanolic extract of Ephedra alata ameliorates cisplatin-induced nephrotoxicity and hepatotoxicity through reducing oxidative stress and genotoxicity. Environ. Sci. Pollut. Res. 2020, 27, 12792–12801. [Google Scholar] [CrossRef]
- Grover, J.; Vats, V.; Rathi, S. Anti-hyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. J. Ethnopharmacol. 2000, 73, 461–470. [Google Scholar] [CrossRef]
- Obatomi, D.K.; Bikomo, E.O.; Temple, V. Anti-diabetic properties of the African mistletoe in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 1994, 43, 13–17. [Google Scholar] [CrossRef]
- Peungvicha, P.; Thirawarapan, S.S.; Temsiririrkkul, R.; Watanabe, H.; Prasain, J.K.; Kadota, S. Hypoglycemic effect of the water extract of Piper sarmentosum in rats. J. Ethnopharmacol. 1998, 60, 27–32. [Google Scholar] [CrossRef]
- Buraczynska, M.; Ksiazek, P.; Baranowicz-Gaszczyk, I.; Jozwiak, L. Association of the VEGF gene polymorphism with diabetic retinopathy in type 2 diabetes patients. Nephrol. Dial. Transplant. 2007, 22, 827–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesterheim, P.; Saitovitch, D.; Staub, H.L. Type 1 diabetes mellitus: Multifactors that confer susceptibility to the autoimmune pathogenesis. Sci. Med. 2007, 17, 218–224. [Google Scholar]
- Dogan, Y.; Akarsu, S.; Ustundag, B.; Yilmaz, E.; Gurgoze, M.K. Serum IL-1β, IL-2, and IL-6 in inslin-dependent diabetic children. Mediat. Inflamm. 2006, 1, 59206. [Google Scholar] [CrossRef] [Green Version]
- Tarawneh, K.A.; Al-Tawarah, N.M.; Abdel-Ghani, A.H.; Al-Majali, A.M.; Khleifat, K.M. Characterization of verotoxigenic Escherichia coli (VTEC) isolates from faeces of small ruminants and environmental samples in Southern Jordan. J. Basic Microbiol. 2009, 49, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Olatunji, O.J.; Zhou, Y.; Hou, X. Allium tuberosum: Antidiabetic and hepatoprotective activities. Food Res. Int. 2017, 102, 681–689. [Google Scholar] [CrossRef]
- Wickramasinghe, A.S.D.; Kalansuriya, P.; Attanayake, A.P. Herbal Medicines Targeting the Improved β-Cell Functions and β-Cell Regeneration for the Management of Diabetes Mellitus. Evid. Based Complementary Altern. Med. 2021, 2021, 2920530. [Google Scholar] [CrossRef]
- Kaczmarczyk-Sedlak, I.; Folwarczna, J.; Sedlak, L.; Zych, M.; Wojnar, W.; Szumińska, I.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E. Effect of caffeine on biomarkers of oxidative stress in lenses of rats with streptozotocin-induced diabetes. Arch. Med. Sci. AMS 2019, 15, 1073. [Google Scholar] [CrossRef]
- Ismail, N.A.; Okasha, S.H.; Dhawan, A.; Abdel-Rahman, A.O.; Shaker, O.G.; Sadik, N.A. Antioxidant enzyme activities in hepatic tissue from children with chronic cholestatic liver disease. Saudi J. Gastroenterol. 2010, 16, 90. [Google Scholar] [CrossRef]
- Sharma, S.; Bansal, N. D-Limonene ameliorates diabetic neuropathic pain in rats. Int. J. Med. Health Res. 2016, 2, 34–49. [Google Scholar]
- Abdullah, K.M.; Arefeen, A.; Shamsi, A.; Alhumaydhi, F.A.; Naseem, I. Insight into the In vitro antiglycation and in vivo antidiabetic effects of thiamine: Implications of vitamin B1 in controlling diabetes. ACS Omega 2021, 6, 12605–12614. [Google Scholar] [CrossRef]










| Compound | MS Fragmentation Pattern | M.W | RT | % | |
|---|---|---|---|---|---|
| 1 | Kaempferol | 285, 213, 229 | 286.24 | 42 | 6.2 |
| 2 | Naringenin | 151, 177, 107 | 272.25 | 15.2 | 2.8 |
| 3 | Kaempferol-3-rhamnoside | 285, 431.1, 284.03, 255, 227 | 432.4 | 33.8 | 2.3 |
| 4 | Quercetin | 257, 229, 285 | 302.23 | 11.7 | 2.2 |
| 5 | Ferulic acid | 149, 134, 178 | 194.18 | 26 | 2 |
| 6 | Epicatechin | 123, 139, 165 | 290.27 | 15.3 | 1.6 |
| 7 | ρ-Hydroxybenzoic acid | 95.1, 121.1, 98 | 138.12 | 27 | 1.57 |
| 8 | Luteolin | 285, 241, 175 | 286.24 | 30.0 | 1.4 |
| 9 | Catechin | 139, 165, 123 | 290.27 | 31.3 | 1.35 |
| 10 | Epigallocatechin | 305.06, 306.07, 303.05, 304.05, 275.05 | 306.27 | 36 | 1.3 |
| 11 | Quercetin-3-glucoside | 465.1, 304.05, 301.03, 305.05 | 464.4 | 28.3 | 1.25 |
| 12 | Gallocatechin | 125, 137, 109, 139, 124 | 306.27 | 40.5 | 1.25 |
| 3 | Hesperidin | 301.07, 302.07, 286.04, 257.08, 325.07 | 610.6 | 41.3 | 1.25 |
| 14 | Epiafzelechin | 97.02, 273.07, 205.08, 137.02, 189.05 | 274.27 | 39.2 | 1.25 |
| 15 | Vitexin | 415.1, 397.1, 367.1 | 432.4 | 23 | 1.2 |
| 16 | Herbacetin | 303, 169, 257 | 302.23 | 13.5 | 1.19 |
| 17 | Luteolin-7-glucoside | 447.09, 285.04, 284.02, 284.05, 447.2 | 448.4 | 10.1 | 1.15 |
| 18 | Isovitexin | 415.1, 367.1, 313 | 432.4 | 17.5 | 1.1 |
| Total | 32.36% |
| Compound | MS Fragmentation Pattern | M.W | RT | % | |
|---|---|---|---|---|---|
| 1 | Limonene | 93.9, 68.6, 136.6, 121.3, 67.3 | 136.23 | 48 | 6.3 |
| 2 | Stearic acid | 265.4, 283.4, 266.5 | 284.5 | 45 | 5.9 |
| 3 | β-Sitosterol | 43, 414, 41, 55, 57, 107 | 414.7 | 35 | 5.5 |
| 4 | Thiamine | 263.1, 233.2, 147.1, 171.1, 58.9 | 265.36 | 10 | 4.1 |
| 5 | Riboflavin | 243, 359, 282 | 376.4 | 31.5 | 3.1 |
| 6 | Quinaldic acid | 174.05, 128.04, 175.05, 156.04 | 173.17 | 9.5 | 1.74 |
| 7 | Kynurenic acid | 171, 190, 144 | 189.17 | 7 | 1.7 |
| 8 | Benzoic acid | 79.05, 123.04, 105.03, 77.03, 106.03 | 122.12 | 6.5 | 1.7 |
| 9 | Hexadecanoic acid | 237.3, 255.3, 227.1 | 256.42 | 25.3 | 1.7 |
| 10 | γ-Terpinene | 93, 91, 121, 136, 77, 79 | 136.23 | 46.5 | 1.65 |
| 11 | Vanillic acid | 125, 93.1, 151.1 | 168.15 | 21 | 1.64 |
| 12 | D-Norpseudoephedrine | 134, 117, 115, 91, 119 | 151.21 | 13.8 | 1.6 |
| 13 | Trans-aconitic acid | 157.1, 133.1, 143.1 | 174.11 | 24 | 1.59 |
| 14 | Nonacosanol | 43, 42, 44 | 424.8 | 32 | 1.57 |
| 15 | Linolenic acid | 279.23, 261.21, 243.21, 95.08, 81.06 | 278.4 | 8.5 | 1.54 |
| 16 | Ascorbic acid | 87, 69, 147, 113,85 | 176.12 | 20.5 | 1.5 |
| 17 | Malic acid | 115, 87.1, 71.1 | 134.09 | 20 | 1.5 |
| 18 | Fumaric acid | 98.9, 45.68, 99.39, 116.35, 52.33 | 116.07 | 24.4 | 1.5 |
| 19 | Oxalic acid | 69.9, 68.23, 89.49 | 90.03 | 16.7 | 1.45 |
| 20 | ρ-Coumaric acid | 147.1, 123.2, 121.2 | 164.16 | 16.2 | 1.45 |
| 21 | Cinnamic acid | 131.1, 131.9 | 148.16 | 14.5 | 1.42 |
| 22 | Dibutyl phthalate | 205, 149, 204 | 278.34 | 32.5 | 1.35 |
| 23 | D-Pseudoephedrine | 91.12, 91.5, 114.8, 132.1, 65.3 | 165.23 | 33.1 | 1.3 |
| 24 | Epigallocatechin | 305.06, 306.07, 303.05, 304.05, 275.05 | 306.27 | 36 | 1.3 |
| 25 | Citric acid | 175, 147.1, 170.1 | 192.12 | 37.9 | 1.3 |
| 26 | Caffeic acid | 135.1, 135.9 | 180.16 | 40.0 | 1.3 |
| 27 | Chlorogenic acid | 191.05, 192.05, 93, 173.04, 353.1 | 354.31 | 43.5 | 1.25 |
| 28 | Linalool | 71.04, 95.08, 81.07, 69.07, 57.07 | 154.25 | 28.5 | 1.25 |
| 29 | γ-Eudesmol | 189, 204, 161,59 | 222.36 | 39.2 | 1.25 |
| 30 | L-Ephedrine | 166, 148, 167 | 165.23 | 22.5 | 1.2 |
| 31 | 1,8-cineole | 43 | 212.28 | 18.0 | 1.15 |
| 32 | Niacin | 106.1, 80 | 123.11 | 21.5 | 1.1 |
| Total | 63.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajleh, M.N.A.; Khleifat, K.M.; Alqaraleh, M.; Al-Hraishat, E.; Al-limoun, M.O.; Qaralleh, H.; Al-Dujaili, E.A.S. Antioxidant and Antihyperglycemic Effects of Ephedra foeminea Aqueous Extract in Streptozotocin-Induced Diabetic Rats. Nutrients 2022, 14, 2338. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14112338
Hajleh MNA, Khleifat KM, Alqaraleh M, Al-Hraishat E, Al-limoun MO, Qaralleh H, Al-Dujaili EAS. Antioxidant and Antihyperglycemic Effects of Ephedra foeminea Aqueous Extract in Streptozotocin-Induced Diabetic Rats. Nutrients. 2022; 14(11):2338. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14112338
Chicago/Turabian StyleHajleh, Maha N. Abu, Khaled M. Khleifat, Moath Alqaraleh, Esra’a Al-Hraishat, Muhamad O. Al-limoun, Haitham Qaralleh, and Emad A. S. Al-Dujaili. 2022. "Antioxidant and Antihyperglycemic Effects of Ephedra foeminea Aqueous Extract in Streptozotocin-Induced Diabetic Rats" Nutrients 14, no. 11: 2338. https://0-doi-org.brum.beds.ac.uk/10.3390/nu14112338