Maltese Mushroom (Cynomorium coccineum L.) as Source of Oil with Potential Anticancer Activity
Abstract
:1. Introduction

2. Experimental Section
2.1. Chemicals
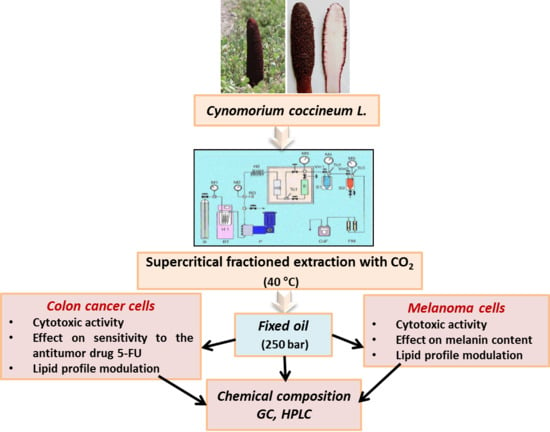
2.2. Plant Materials and Fixed Oil Extraction
2.3. Tumor Cell Cultures
2.4. Cytotoxic Activity of Fixed Oil: MTT Assay
2.5. Effect of Oil on Cytotoxic Activity of 5-FU in Cancer Caco-2 Cells: MTT Assay
2.6. Fatty Acid Profile Modulation in Cancer Caco-2 and B16F10 Melanoma Cells
2.7. Extraction and Separation of Lipid Components in Fixed Oil and Cancer Cells
2.8. Analyses of Lipid Components in Fixed Oil and Cancer Cells
2.9. Effect of Oil on Melanin Production in B16F10 Melanoma Cells
2.10. Statistical Analyses
3. Results
3.1. Composition of C. Coccineum Fixed Oil
| Fatty Acid | C. coccineum Oil | Caco-2 Cells | B16F10 Cells |
|---|---|---|---|
| 12:0 | 2.00 ± 0.33 | 0.28 ± 0.09 | trace |
| 14:0 | 4.40 ± 0.92 | 2.12 ± 0.21 | 2.36 ± 0.61 |
| 16:0 | 15.75 ± 1.10 | 19.84 ± 2.52 | 24.37 ± 2.09 |
| 16:1n-7 | 2.39 ± 0.51 | 3.86 ± 1.23 | 6.59 ± 0.07 |
| 18:0 | 2.17 ± 0.16 | 14.34 ± 3.67 | 7.47 ± 0.39 |
| 18:1n-7 | 0.85 ± 0.15 | 4.85 ± 1.07 | 5.34 ± 0.33 |
| 18:1n-9 | 37.03 ± 3.90 | 17.18 ± 2.86 | 28.46 ± 1.61 |
| 18:2n-6 | 19.46 ± 2.51 | 1.67 ± 0.30 | 1.54 ± 0.11 |
| 18:3n-3 | 8.13 ± 1.32 | 0.49 ± 0.01 | trace |
| 20:0 | 2.21 ± 0.22 | 8.72 ± 1.08 | 9.37 ± 3.23 |
| 20:1n-9 | 0.30 ± 0.06 | ||
| 20:3n-3 | trace | trace | |
| 20:3n-6 | 0.79 ± 0.08 | 0.68 ± 0.09 | |
| 20:3n-9 | 2.93 ± 1.57 | 3.06 ± 2.82 | |
| 20:4n-6 | 4.82 ± 0.79 | 0.94 ± 0.12 | |
| 20:5n-6 | 0.84 ± 0.30 | 0.20 ± 0.05 | |
| 22:5n-3 | 2.01 ± 0.44 | 0.58 ± 0.05 | |
| 22:6n-3 | 2.52 ± 0.48 | 0.82 ± 0.23 | |
| SFA | 33.65 ± 0.13 | 48.17 ± 8.33 | 43.57 ± 0.08 |
| MUFA | 44.21 ± 0.18 | 25.89 ± 4.72 | 40.39 ± 1.01 |
| PUFA | 24.13 ± 0.10 | 17.16 ± 3.26 | 7.82 ± 2.73 |
3.2. Cytotoxic Effect of Fixed Oil in Cancer Cells
3.3. Effect of Fixed Oil on Cytotoxic Activity of 5-FU in Cancer Caco-2 Cells: MTT Assay

3.4. Effect of C. Coccineum Fixed Oil on Lipid Profile of Cancer Cells
| Fatty Acid | Caco-2 Cells | B16F10 Cells |
|---|---|---|
| 16:1n-7 | 13.37 ± 4.71 | 17.34 ± 1.57 |
| 18:1n-7 + 18:1n-9 | 57.88 ± 11.19 | 98.34 ± 7.24 |
| 18:2n-6 | 4.29 ± 2.95 | 2.80 ± 1.05 |
| 18:3n-3 | 0.23 ± 0.12 | 0.26 ± 0.08 |
| 20:3n-3 | trace | trace |
| 20:3n-6 | 1.55 ± 0.58 | 2.77 ± 0.00 |
| 20:3n-9 | 0.95 ± 0.19 | 3.29 ± 0.27 |
| 20:4n-6 | 4.84 ± 0.86 | 4.75 ± 0.35 |
| 20:5n-3 | 1.16 ± 0.33 | 0.75 ± 0.06 |
| 22:4n-6 | 0.38 ± 0.09 | 0.33 ± 0.04 |
| 22:5n-3 | 2.19 ± 0.55 | 3.44 ± 0.21 |
| 22:6n-3 | 4.05 ± 0.98 | 4.17 ± 0.44 |


3.5. Effect of Fixed Oil on Melanin Production in B16F10 Melanoma Cells

4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- IUCN; International Union for Conservation of Nature and Natural Resources; Centre for Mediterranean Cooperation. Cynomorium coccineum L. In A Guide to Medicinal Plants in North Africa; IUCN Centre for Mediterranean Cooperation: Malaga, Spain, 2005; pp. 99–100. [Google Scholar]
- Dharmananda, S. Cynomorium—Parasitic Plant Widely Used in Traditional Medicine. Available online: http://www.itmonline.org/arts/cynomorium.htm (accessed on 8 January 2013).
- Abd El-Rahman, H.A.; El-Badry, A.A.; Mahmoud, O.M.; Harraz, F.A. The effect of the aqueous extract of Cynomorium coccineum on the epididymal sperm pattern of the rat. Phytother. Res. 1999, 13, 248–250. [Google Scholar]
- Duke, J.A.; Duke, P.-A.K.; duCellier, J.L. Dukeʼs Handbook of Medicinal Plants of the Bible, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Lebling, R.W. The treasure of tarthuth. Saudi Aramco World 2003, 54, 12–17. [Google Scholar]
- Abdel-Magied, E.M.; Abdel-Rahman, H.A.; Harraz, F.M. The effect of aqueous extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature Wistar rats. J. Ethnopharmacol. 2001, 75, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Al-Qarawi, A.A.; Abdel-Rahman, H.A.; El-Badry, A.A.; Harraz, F.; Razig, N.A.; Abdel-Magied, E.M. The effect of extracts of Cynomorium coccineum and Withania somnifera on gonadotrophins and ovarian follicles of immature wistar rats. Phytother. Res. 2000, 14, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Rached, W.; Benamar, H.; Bennaceur, M.; Marouf, A. Screening of the antioxidant potential of some Algerian indigenous plants. J. Biol. Sci. 2010, 10, 316–324. [Google Scholar] [CrossRef]
- Ikram, M.; Sar, M.S.; Fakouhi, T. Hypotensive agent from Cynomorium coccineum. Pahlavi Med. J. 1978, 9, 167–181. [Google Scholar] [PubMed]
- Zucca, P.; Rosa, A.; Tuberoso, C.I.G.; Piras, A.; Rinaldi, A.C.; Sanjust, E.; Dessì, M.A.; Rescigno, A. Evaluation of antioxidant potential of “Maltese Mushroom” (Cynomorium coccineum) by means of multiple chemical and biological assays. Nutrients 2013, 5, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.; Guo, Z.; Miao, J.; Wang, Z.; Li, Q.; Chai, X.; Li, M. The genus Cynomorium in China: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2013, 147, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Rescigno, A.; Piras, A.; Atzeri, A.; Scano, P.; Porcedda, S.; Zucca, P.; Dessì, M.A. Chemical composition and effect on intestinal Caco-2 cell viability and lipid profile of fixed oil from Cynomorium coccineum L. Food Chem. Toxicol. 2012, 50, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Al Ashaal, H.A.; Farghaly, A.A.; Abd El Aziz, M.M.; Ali, M.A. Phytochemical investigation and medicinal evaluation of fixed oil of Balanites aegyptiaca fruits (Balantiaceae). J. Ethnopharmacol. 2010, 127, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Naqshbandi, A.; Rizwan, S.; Khan, F. Dietary supplementation of flaxseed oil ameliorates the effect of cisplatin on rat kidney. J. Funct. Foods 2013, 5, 316–326. [Google Scholar] [CrossRef]
- Singh, S.; Taneja, M.; Majumdar, D.K. Biological activities of Ocimum sanctum L. fixed oil—An overview. Indian J. Exp. Biol. 2007, 45, 403–412. [Google Scholar] [PubMed]
- Sala-Vila, A.; Folkes, J.; Calder, P.C. The effect of three lipid emulsions differing in fatty acid composition on growth, apoptosis and cell cycle arrest in the HT-29 colorectal cancer cell line. Clin. Nutr. 2010, 29, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Han, E.S.; Park, D.K. The ethyl acetate extract of PGP (Phellinus linteus grown on Panax ginseng) suppresses B16F10 melanoma cell proliferation through inducing cellular differentiation and apoptosis. J. Ethnopharmacol. 2010, 132, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.K.; Ho, C.J.; Lia, S.C.; Yanga, S.H.; Hou, W.C.; Cheng, H.H. Preventive effects of rice bran oil on 1,2-dimethylhydrazine/dextran sodium sulphate-induced colon carcinogenesis in rats. Food Chem. 2011, 126, 562–567. [Google Scholar] [CrossRef]
- Rajasekar, S.; Park, D.J.; Park, C.; Park, S.; Park, Y.H.; Kim, S.T.; Choi, Y.H.; Choi, Y.W. In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma. J. Ethnopharmacol. 2012, 144, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Giermasz, A.; Nowis, D.; Jalili, A.; Basak, G.; Marczak, M.; Makowski, M.; Czajka, A.; Młynarczuk, I.; Hoser, G.; Stok osa, T.; Lewandowski, S.; Jakóbisiak, M. Antitumor activity of tributyrin in murine melanoma model. Cancer Lett 2001, 164, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.; de Lima, T.M.; Curi, R.; Castrucci, A.M. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicol. Vitro 2005, 19, 553–560. [Google Scholar] [CrossRef]
- Jordan, A.; Stein, J. Effect of an omega-3 fatty acid containing lipid emulsion alone and in combination with 5-fluorouracil (5-FU) on growth of the colon cancer cell line Caco-2. Eur. J. Nutr. 2003, 42, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Travelin, S.; Gråsjö, J.; Taipalensuu, J.; Ocklind, G.; Artursson, P. Application of epithelial cell culture in studies of drug transpor. In Epithelial Cell Culture Protocols; Wise, C., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 233–272. [Google Scholar]
- Schiller, C.J.; Klainz, A.; Mynett, K.; Gescher, A. Assessment of viability of hepatocytes in suspension using the MTT assay. Toxicol. Vitro 1992, 6, 575–578. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In Advantage in Lipid Methodology–Two; Christie, W.W., Ed.; The Oily Press: Dundee, Scotland, 1993; pp. 69–111. [Google Scholar]
- Soddu, G.; Sanjust, E.; Murgia, S.; Rescigno, A. Interference of some tryptophan metabolites in the formation of melanin in vitro. Pigm. Cell. Res. 2004, 5, 135–141. [Google Scholar] [CrossRef]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jerónimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Scano, P.; Atzeri, A.; Deiana, M.; Falchi, A.M. Potential anti-tumor effects of Mugil cephalus processed roe extracts on colon cancer cells. Food Chem. Toxicol. 2013, 60, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Pereira-Caro, G.; Bacon, J.R.; Bongaerts, R.; Sarria, B.; Bravo, L.; Kroon, P.A. Anticancer activity of olive oil hydroxytyrosyl acetate in human adenocarcinoma Caco-2 cells. J. Agric. Food Chem. 2013, 61, 3264–3269. [Google Scholar] [CrossRef] [PubMed]
- Tabolacci, C.; Lentini, A.; Mattioli, P.; Provenzano, B.; Oliverio, S.; Carlomosti, F.; Beninati, S. Antitumor properties of aloe-emodin and induction of transglutaminase 2 activity in B16–F10 melanoma cells. Life Sci. 2010, 87, 316–324. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.L.M.; Nunes-Pinheiro, D.C.; Tomé, A.R.; Mota, E.F.; Lima-Verde, I.A.; Pinheiro, F.G.; Campello, C.C.; de Morais, S.M. In vivo topical anti-inflammatory and wound healing activities of the fixed oil of Caryocar coriaceum Wittm. seeds. J. Ethnopharmacol. 2010, 129, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.; Jones, P.J. Evidence of health benefits of canola oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Stavro, P.M.; Thompson, L.U. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr. Cancer 2002, 43, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, J.; Thompson, L.U. The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenografts is attributed to both its lignan and oil components. Int. J. Cancer 2005, 116, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.R.; Edwards, M.J.; DeFilipis, A.P.; Jacobson, T.A. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J. Nutr. 2006, 136, 83–87. [Google Scholar] [PubMed]
- Biondo, P.D.; Brindley, D.N.; Sawyer, M.B.; Field, C.J. The potential for treatment with dietary long-chain polyunsaturated n-3 fatty acids during chemotherapy. J. Nutr. Biochem. 2008, 19, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Roynette, C.E.; Calder, P.C.; Dupertuis, Y.M.; Pichard, C. n-3 polyunsaturated fatty acids and colon cancer prevention. Clin. Nutr. 2004, 23, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, A.; Nieddu, M.; Piras, A.; Atzeri, A.; Putzu, D.; Rescigno, A. Maltese Mushroom (Cynomorium coccineum L.) as Source of Oil with Potential Anticancer Activity. Nutrients 2015, 7, 849-864. https://0-doi-org.brum.beds.ac.uk/10.3390/nu7020849
Rosa A, Nieddu M, Piras A, Atzeri A, Putzu D, Rescigno A. Maltese Mushroom (Cynomorium coccineum L.) as Source of Oil with Potential Anticancer Activity. Nutrients. 2015; 7(2):849-864. https://0-doi-org.brum.beds.ac.uk/10.3390/nu7020849
Chicago/Turabian StyleRosa, Antonella, Mariella Nieddu, Alessandra Piras, Angela Atzeri, Danilo Putzu, and Antonio Rescigno. 2015. "Maltese Mushroom (Cynomorium coccineum L.) as Source of Oil with Potential Anticancer Activity" Nutrients 7, no. 2: 849-864. https://0-doi-org.brum.beds.ac.uk/10.3390/nu7020849