Supplementation of Antipsychotic Treatment with the Amino Acid Sarcosine Influences Proton Magnetic Resonance Spectroscopy Parameters in Left Frontal White Matter in Patients with Schizophrenia
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
| Features | Group | p-value | ||
|---|---|---|---|---|
| Sarcosine (n = 25) | Control (n = 25) | |||
| Gender | Female | 14 | 12 | >0.05 |
| Male | 11 | 13 | ||
| Age (mean, year) | 36.5 | 40 | >0.05 | |
| Mean number of hospitalizations | 5 | 4 | >0.05 | |
| Mean duration of the illness (years) | 12.3 | 13.2 | >0.05 | |
| Mean timespan of education per patient (years) | 14.2 | 14.4 | >0.05 | |
| Smoking | 9 | 11 | >0.05 | |
| Antipsychotic treatment (DDD) | 1.94 | 1.97 | >0.05 | |
| Anti-depressive treatment (DDD) | 0.58 | 0.6 | >0.05 | |
| PANSS total (± SD) | 71.4 ± 14 | 73.3 ± 13 | >0.05 | |
2.2. Spectroscopy
- FLAIR (fluid-attenuated inversion recovery) sequences in axial plane with following parameters: Repetition Time (TR), 9000 ms; Echo Time (TE), 105 ms; inversion time (TI), 2500 ms; flip angle, 150°; voxel size 1.4 × 1.3 × 3 mm.
- T2-weighted sequences were obtained in coronal plane with following parameters: TR = 5000 ms; TE = 100 ms; flip angle, 50°; voxel size 0.6 × 0.6 × 5.0 mm.
- T1 weighted sequences in transverse plane with following parameters: TR = 400 ms; TE = 7.8 ms; flip angle, 90° g; voxel size 0.9 × 0.9 × 0.5 mm.

2.3. Statistical Analysis
3. Results
| Compared Ratios | Baseline | After 6 months | Baseline vs. after 6 months | Baseline vs. after 6 months | ||||
|---|---|---|---|---|---|---|---|---|
| Sarcosine (mean ± SD) | Placebo (mean ± SD) | p-value | Sarcosine (mean ± SD) | Placebo (mean ± SD) | p-value | Sarcosine p-value | Placebo p-value | |
| NAA/Cr | 1.96 (2.55) | 2.43 (1.79) | >0.05 | 2.37 (0.53) | 2.75 (1.37) | >0.05 | 0.0048 | >0.05 |
| Cho/Cr | 1.31 (0.87) | 1.21 (0.84) | >0.05 | 1.02 (0.34) | 1.05 (0.86) | >0.05 | >0.05 | >0.05 |
| mI/Cr | 0.28 (0.18) | 0.37 (0.28) | >0.05 | 0.28 (0.15) | 0.30 (0.14) | >0.05 | >0.05 | >0.05 |
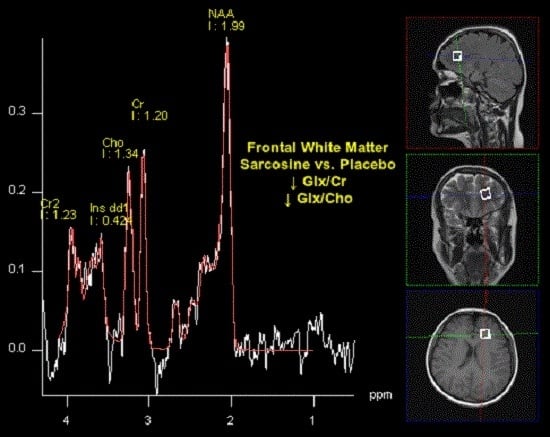
| Glx/Cr | 0.73 (0.33) | 0.75 (0.20) | >0.05 | 0.51 (0.21) | 0.80 (0.20) | 0.0062 | 0.0281 | >0.05 |
| NAA/Cho | 2.34 (0.84) | 2.18 (0.83) | >0.05 | 2.30 (1.72) | 2.65 (2.40) | >0.05 | >0.05 | >0.05 |
| mI/Cho | 0.27 (0.25) | 0.30 (0.23) | >0.05 | 0.27 (0.15) | 0.31 (0.13) | >0.05 | >0.05 | >0.05 |
| Glx/Cho | 0.75 (0.27) | 0.83 (0.20) | >0.05 | 0.57 (0.18) | 0.85 (0.20) | 0.0041 | >0.05 | >0.05 |
| Difference in Concentration Ratio | Predictor | β—Coefficient (± SD) | Corrected R2 of the Model | p Value |
|---|---|---|---|---|
| NAA/Cr | Age | −0.0234 (0.0097) | 0.1565 | 0.0208 * |
| Smoking | 0.3629 (0.1978) | 0.0733 | ||
| Glx/Cr | Group | 0.2079 (0.0616) | 0.1844 | 0.0015 * |
| NAA/Cho | Age | 0.02679 (0.0194) | 0.0192 | 0.1748 |
| mI/Cho | Age | 0.0038 (0.0018) | 0.0705 | 0.0396 * |
| Glx/Cho | Group | 0.12943 (0.0562) | 0.1207 | 0.0260 * |
| Smoking | −0.09086 (0.0565) | 0.1150 |
4. Discussion
4.1. Axonal Pathology
4.2. Astroglia Pathology
4.3. Olidendroglia Pathology
4.4. Analysis of Spectroscopic Parameters
4.4.1. Glx
4.4.2. NAA
4.4.3. mI
4.5. Limitations of the Study
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry 1991, 148, 1474–1486. [Google Scholar] [PubMed]
- Aghajanian, G.K.; Marek, G.J. Serotonin model of schizophrenia: Emerging role of glutamate mechanisms. Brain Res. Brain Res. Rev. 2000, 31, 302–312. [Google Scholar] [CrossRef]
- Carlsson, A.; Waters, N.; Waters, S.; Carlsson, M.L. Network interactions in schizophrenia—Therapeutic implications. Brain Res. Brain Res. Rev. 2000, 31, 342–349. [Google Scholar] [CrossRef]
- Coyle, J.T. The glutamatergic dysfunction hypothesis for schizophrenia. Harv. Rev. Psychiatry 1996, 3, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Newcomer, J.W.; Farber, N.B. NMDA receptor hypofunction model of schizophrenia. J. Psychiatr. Res. 1999, 33, 523–533. [Google Scholar] [CrossRef]
- Coyle, J.T.; Basu, A.; Benneyworth, M.; Balu, D.; Konopaske, G. Glutamatergic synaptic dysregulation in schizophrenia: Therapeutic implications. Handb. Exp. Pharmacol. 2012, 213, 267–295. [Google Scholar] [PubMed]
- Allen, R.M.; Young, S.J. Phencyclidine-induced psychosis. Am. J. Psychiatry 1978, 135, 1081–1084. [Google Scholar] [PubMed]
- Lahti, A.C.; Weiler, M.A.; Tamara Michaelidis, B.A.; Parwani, A.; Tamminga, C.A. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 2001, 25, 455–467. [Google Scholar] [CrossRef]
- Lahti, A.C.; Koffel, B.; LaPorte, D.; Tamminga, C.A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995, 13, 9–19. [Google Scholar] [CrossRef]
- Karp, H.N.; Kaufman, N.D.; Anand, S.K. Phencyclidine poisoning in young children. J. Pediatr. 1980, 97, 1006–1009. [Google Scholar] [CrossRef]
- Jääskeläinen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr. Bull. 2013, 39, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs 2011, 25, 859–885. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.E.; Lin, P.Y. Strategies to enhance N-methyl-d-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr. Pharm. Des. 2010, 16, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C. Glycine transport inhibitors in the treatment of schizophrenia. Handb. Exp. Pharmacol. 2012, 213, 367–399. [Google Scholar] [CrossRef] [PubMed]
- Kantrowitz, J.; Javitt, D.C. Glutamatergic transmission in schizophrenia: From basic research to clinical practice. Curr. Opin. Psychiatry 2012, 25, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Strzelecki, D.; Rabe-Jabłońska, J. Changes in positive and negative symptoms, general psychopathology in schizophrenic patients during augmentation of antipsychotics with glycine: A preliminary 10-week open-label study. Psychiatr. Pol. 2011, 45, 825–837. [Google Scholar] [PubMed]
- Alberati, D.; Moreau, J.L.; Lengyel, J.; Hauser, N.; Mory, R.; Borroni, E.; Pinard, E.; Knoflach, F.; Schlotterbeck, G.; Hainzl, D.; et al. Glycine reuptake inhibitor RG1678: A pharmacologic characterization of an investigational agent for the treatment of schizophrenia. Neuropharmacology 2012, 62, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.E.; Fox, S.D.; Issaq, H.J.; Xu, X.; Chu, L.W.; Veenstra, T.D.; Hsing, A.W. A reproducible and high-throughput HPLC/MS method to separate sarcosine from α- and β-alanine and to quantify sarcosine in human serum and urine. Anal. Chem. 2011, 83, 5735–40. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.H.; Stabler, S.P.; Lindenbaum, J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism 1993, 42, 1448–1460. [Google Scholar] [CrossRef]
- Harsing, L.G., Jr.; Juranyi, Z.; Gacsalyi, I.; Tapolcsanyi, P.; Czompa, A.; Matyus, P. Glycine transporter type-1 and its inhibitors. Curr. Med. Chem. 2006, 13, 1017–1044. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.G.; Masuoka, T.; Gong, X.D.; Chen, K.S.; Yanagawa, Y.; Law, S.K.; Konishi, S. NMDA receptor activation enhances inhibitory GABAergic transmission onto hippocampal pyramidal neurons via presynaptic and postsynaptic mechanisms. J. Neurophysiol. 2011, 105, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Tsien, R.W.; Goff, D.C.; Halassa, M.M. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr. Res. 2015, 167, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Jodo, E. The role of the hippocampo-prefrontal cortex system in phencyclidine-induced psychosis: A model for schizophrenia. J. Physiol. Paris 2013, 107, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Homayoun, H.; Moghaddam, B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 2007, 27, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Greene, R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus 2001, 11, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.T.; Berk, M.; Lucas, M.D. A neuropsychological study of prefrontal lobe function in the positive and negative subtypes of schizophrenia. J. Genet. Psychol. 1997, 158, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Tuan, T.A.; Woon, P.S.; Abdul-Rahman, M.F.; Graham, S.; Sim, K. Hippocampal-cortical structural connectivity disruptions in schizophrenia: An integrated perspective from hippocampal shape, cortical thickness, and integrity of white matter bundles. Neuroimage 2010, 52, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.M.; Lappin, J.; Di Forti, M. Schizophrenia: From developmental deviance to dopamine dysregulation. Eur. Neuropsychopharmacol. 2008, 18, S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.J. Schizophrenia susceptibility genes converge on interlinked pathways related to glutamatergic transmission and long-term potentiation, oxidative stress and oligodendrocyte viability. Schizophr. Res. 2006, 86, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stys, P.K.; Lipton, S.A. White matter NMDA receptors: An unexpected new therapeutic target? Trends Pharmacol. Sci. 2007, 28, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Hakak, Y.; Walker, J.R.; Li, C.; Wong, W.H.; Davis, K.L.; Buxbaum, J.D.; Haroutunian, V.; Fienberg, A.A. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 4746–4751. [Google Scholar] [CrossRef] [PubMed]
- Uranova, N.; Orlovskaya, D.; Vikhreva, O.; Zimina, I.; Kolomeets, N.; Vostrikov, V.; Rachmanova, V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res. Bull. 2001, 55, 597–610. [Google Scholar] [CrossRef]
- Uranova, N.A.; Vostrikov, V.M.; Orlovskaya, D.D.; Rachmanova, V.I. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: A study from the Stanley Neuropathology Consortium. Schizophr. Res. 2004, 67, 269–275. [Google Scholar] [CrossRef]
- Hof, P.R.; Haroutunian, V.; Friedrich, V.L., Jr.; Byne, W.; Buitron, C.; Perl, D.P.; Davis, K.L. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol. Psychiatry 2003, 53, 1075–1085. [Google Scholar] [CrossRef]
- Okugawa, G.; Sedvall, G.; Agartz, I. Reduced grey and white matter volumes in the temporal lobe of male patients with chronic schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2002, 252, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Takase, K.; Tamagaki, C.; Okugawa, G.; Nobuhara, K.; Minami, T.; Sugimoto, T.; Sawada, S.; Kinoshita, T. Reduced white matter volume of the caudate nucleus in patients with schizophrenia. Neuropsychobiology 2004, 50, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Hulshoff Pol, H.; Brans, R.; van Haren, N.; Schnack, H.; Langen, M.; Baare, W.; van Oel, C.J.; Kahn, R.S. Gray and white matter volume abnormalities in monozygotic and same-gender dizygotic twins discordant for schizophrenia. Biol. Psychiatry 2004, 55, 126–130. [Google Scholar] [CrossRef]
- Hulshoff Pol, H.; Schnack, H.; Mandl, R.; Cahn, W.; Collins, D.; Evans, A.; Kahn, R.S. Focal white matter density changes in schizophrenia: Reduced inter-hemispheric connectivity. Neuroimage 2004, 21, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Foong, J.; Symms, M.; Barker, G.; Maier, M.; Woermann, F.; Miller, D.; Ron, M.A. Neuropathological abnormalities in schizophrenia: Evidence from magnetization transfer imaging. Brain 2001, 124, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Bagary, M.; Symms, M.; Barker, G.; Mutsatsa, S.; Joyce, E.; Ron, M. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch. Gen. Psychiatry 2003, 60, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, M.; Tang, C.; Peled, S.; Gudbjartsson, H.; Lu, D.; Hazlett, E.; Downhill, J.; Haznedar, M.; Fallon, J.H.; Atlas, S.W. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport 1998, 9, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Hedehus, M.; Moseley, M.; de Crespigny, A.; Sullivan, E.; Pfefferbaum, A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch. Gen. Psychiatry 1999, 56, 367–394. [Google Scholar] [CrossRef] [PubMed]
- Agartz, I.; Andersson, J.; Skare, S. Abnormal brain white matter in schizophrenia: A diffusion tensor imaging study. Neuroreport 2001, 12, 2251–2254. [Google Scholar] [CrossRef] [PubMed]
- Wolkin, A.; Choi, S.; Szilagyi, S.; Sanfilipo, M.; Rotrosen, J.; Lim, K. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: A diffusion tensor imaging study. Am. J. Psychiatry 2003, 160, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Szeszko, P.; Ardekani, B.; Ashtari, M.; Kumra, S.; Robinson, D.; Sevy, S.; Gunduz-Bruce, H.; Malhotra, A.K.; Kane, J.M.; Bilder, R.M.; et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: A diffusion tensor imaging study. Am. J. Psychiatry 2005, 162, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Friedman, J.; Ernst, T.; Zhong, K.; Tsopelas, N.D.; Davis, K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: Implication for glial dysfunction. Biol. Psychiatry 2007, 62, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, T.; Bouix, S.; Lyall, A.E.; Hosokawa, T.; Saito, Y.; Melonakos, E.; Westin, C.F.; Seidman, L.J.; Goldstein, J.; Mesholam-Gately, R.; et al. Abnormal white matter connections between medial frontal regions predict symptoms in patients with first episode schizophrenia. Cortex 2015, 71, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Kraguljac, N.V.; Reid, M.; White, D.; Jones, R.; den Hollander, J.; Lowman, D.; Lahti, A.C. Neurometabolites in schizophrenia and bipolar disorder—A systematic review and meta-analysis. Psychiatry Res. 2012, 203, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.N.; Namboodiri, A.M. N-acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.R.; Namboodiri, M.A. Differential distribution of N-acetylaspartylglutamate and N-acetylaspartate immunoreactivities in rat forebrain. J. Neurocytol. 1995, 24, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Meyerhoff, D.J.; MacKay, S.; Bachman, L.; Poole, N.; Dillon, W.P.; Weiner, M.W.; Fein, G. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: In vivo 1H magnetic resonance spectroscopic imaging. Neurology 1993, 43, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Sager, T.N.; Topp, S.; Torup, L.; Hanson, L.G.; Egestad, B.; Moller, A. Evaluation of CA1 damage using single-voxel 1H-MRS and un-biased stereology: Can non-invasive measures of N-acetyl-asparate following global ischemia be used as a reliable measure of neuronal damage? Brain Res. 2001, 892, 166–175. [Google Scholar] [CrossRef]
- Szulc, A.; Konarzewska, B.; Galińska-Skok, B.; Lazarczyk, J.; Waszkiewicz, N.; Tarasów, E.; Milewski, R.; Walecki, J. Proton magnetic resonance spectroscopy measures related to short-term symptomatic outcome in chronic schizophrenia. Neurosci. Lett. 2013, 547, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.; Fowler, L.; Whitton, P.S.; Pearce, B. Release of arginine, glutamate and glutamine in the hippocampus of freely moving rats: Involvement of nitric oxide. Brain Res. Bull. 2005, 65, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Schwerk, A.; Alves, F.D.; Pouwels, P.J.; van Amelsvoort, T. Metabolic alterations associated with schizophrenia: A critical evaluation of proton magnetic resonance spectroscopy studies. J. Neurochem. 2014, 128, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Kegeles, L.S.; Mao, X.; Stanford, A.D.; Girgis, R.; Ojeil, N.; Xu, X.; Gil, R.; Slifstein, M.; Abi-Dargham, A.; Lisanby, S.H.; et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry 2012, 69, 449–459. [Google Scholar] [PubMed]
- Kraguljac, N.V.; White, D.M.; Reid, M.A.; Lahti, A.C. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry 2013, 70, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Sandoval, C.; León-Ortiz, P.; Favila, R.; Stephano, S.; Mamo, D.; Ramírez-Bermúdez, J.; Graff-Guerrero, A. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology 2011, 36, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Steen, R.G.; Hamer, R.M.; Lieberman, J.A. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: A systematic review and meta-analysis. Neuropsychopharmacology 2005, 30, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Brugger, S.; Davis, J.M.; Leucht, S.; Stone, J.M. Proton magnetic resonance spectroscopy and illness stage in schizophrenia—A systematic review and meta-analysis. Biol. Psychiatry 2011, 69, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Marsman, A.; van den Heuvel, M.P.; Klomp, D.W.; Kahn, R.S.; Luijten, P.R.; Hulshoff Pol, H.E. Glutamate in schizophrenia: A focused review and meta-analysis of ¹H-MRS studies. Schizophr. Bull. 2013, 39, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Dallal, G.E. Randomization. Available online: http://www.randomization.com (accessed on 6 October 2012).
- Kay, S.R.; Fiszbein, A.; Opfer, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Song, S.K.; Sun, S.W.; Ramsbottom, M.J.; Chang, C.; Russell, J.; Cross, A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002, 17, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Holleran, L.; Ahmed, M.; Anderson-Schmidt, H.; McFarland, J.; Emsell, L.; Leemans, A.; Scanlon, C.; Dockery, P.; McCarthy, P.; Barker, G.J.; et al. Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology 2014, 39, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Mangin, J.F.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging. 2001, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.D.; Mori, S.; Leemans, A. Diffusion tensor imaging and beyond. Magn. Reson. Med. 2011, 65, 1532–1556. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Ohnishi, T.; Hashimoto, R.; Nemoto, K.; Moriguchi, Y.; Noguchi, H.; Nakabayashi, T.; Hori, H.; Harada, S.; Saitoh, O.; et al. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res. 2007, 154, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Rotarska-Jagiela, A.; Oertel-Knoechel, V.; DeMartino, F.; van de Ven, V.; Formisano, E.; Roebroeck, A.; Rami, A.; Schoenmeyer, R.; Haenschel, C.; Hendler, T.; et al. Anatomical brain connectivity and positive symptoms of schizophrenia: A diffusion tensor imaging study. Psychiatry Res. 2009, 174, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schlösser, R.G.; Nenadic, I.; Wagner, G.; Güllmar, D.; von Consbruch, K.; Köhler, S.; Schultz, C.C.; Koch, K.; Fitzek, C.; Matthews, P.M.; et al. White matter abnormalities and brain activation in schizophrenia: A combined DTI and fMRI study. Schizophr. Res. 2007, 89, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shergill, S.S.; Kanaan, R.A.; Chitnis, X.A.; O’Daly, O.; Jones, D.K.; Frangou, S.; Williams, S.C.; Howard, R.J.; Barker, G.J.; Murray, R.M.; et al. A diffusion tensor imaging study of fasciculi in schizophrenia. Am. J. Psychiatry 2007, 164, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sussmann, J.E.; Lymer, G.K.; McKirdy, J.; Moorhead, T.W.; Muñoz Maniega, S.; Job, D.; Hall, J.; Bastin, M.E.; Johnstone, E.C.; Lawrie, S.M.; et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.I.; Tang, C.; Carpenter, D.; Buchsbaum, M.; Schmeidler, J.; Flanagan, L.; Golembo, S.; Kanellopoulou, I.; Ng, J.; Hof, P.R.; et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am. J. Psychiatry 2008, 165, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Ouyang, X.; Tao, H.; Liu, H.; Li, L.; Zhao, J.; Xue, Z.; Wang, F.; Jiang, S.; Shan, B.; et al. Complementary diffusion tensor imaging study of the corpus callosum in patients with first-episode and chronic schizophrenia. J. Psychiatry Neurosci. 2011, 36, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.C.; Andreasen, N.C.; Nopoulos, P.; Arndt, S.; Magnotta, V.; Flaum, M. Progressive structural brain abnormalities and their relationship to clinical outcome: A longitudinal magnetic resonance imaging study early in schizophrenia. Arch. Gen. Psychiatry 2003, 60, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. Tripartite synapses: Roles for astrocytic purins in the control of synaptic physiology and behavior. Neuropharmacology 2009, 57, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Karlsgodt, K.H.; Jacobson, S.C.; Seal, S.C.; Fusar-Poli, P. The relationship of developmental changes in white matter to the onset of psychosis. Curr. Pharm. Des. 2012, 18, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Sakurai, T.; Davis, K.L.; Buxbaum, J.D. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog. Neurobiol. 2011, 93, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Byne, W.; Tatusov, A.; Yiannoulos, G.; Vong, G.S.; Marcus, S. Effects of mental illness and aging in two thalamic nuclei. Schizophr. Res. 2008, 106, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.K.; Uylings, H.B.; Sanz-Arigita, E.; Pakkenberg, B. Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am. J. Psychiatry 2004, 161, 882–888. [Google Scholar] [PubMed]
- Uranova, N.A.; Vostrikov, V.M.; Vikhreva, O.V.; Zimina, I.S.; Kolomeets, N.S.; Orlovskaya, D.D. The role of oligodendrocyte pathology in schizophrenia. Int. J. Neuropsychopharmacol. 2007, 10, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Katsel, P.; Davis, K.L.; Haroutunian, V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: A gene ontology study. Schizophr. Res. 2005, 79, 157–73. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.G.; Steiner, J.; Guest, P.C.; Dobrowolny, H.; Bogerts, B. Glial cells as key players in schizophrenia pathology: Recent insights and concepts of therapy. Schizophr. Res. 2015, 161, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Grace, A.A. The dopamine system and the pathophysiology of schizophrenia: A basic science perspective. Int. Rev. Neurobiol. 2007, 78, 41–68. [Google Scholar] [PubMed]
- Olney, J.W.; Farber, N.B. Glutamate receptor dysfunction and schizophrenia. Arch. Gen. Psychiatry 1995, 52, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Matute, C.; Domercq, M.; Sanchez-Gomez, M.V. Glutamate-mediated glial injury: Mechanisms and clinical importance. Glia 2006, 53, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Cooper, A.J.; Thida, T.; Shinn, A.K.; Cohen, B.M.; Ongür, D. Myelin and axon abnormalities in schizophrenia measured with magnetic resonance imaging techniques. Biol. Psychiatry 2013, 74, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Ross, B.D.; Bluml, S. Direct determination of the N-acetyl-l-aspartate synthesis rate in the human brain by 13C MRS and [1–13C] glucose infusion. J. Neurochem. 2001, 77, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.; Flashman, L.; Flaum, M.; Arndt, S.; Swayze, V., 2nd.; O’Leary, D.; Ehrhardt, J.C.; Yuh, W.T. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA 1994, 272, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, G.; Selemon, L.; Goldman-Rakic, P. Neuronal and glial somal size in the prefrontal cortex: A postmortem morphometric study of schizophrenia and Huntington disease. Arch. Gen. Psychiatry 1998, 55, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Colter, N.; Corsellis, J.; Crow, T.; Frith, C.; Jagoe, R.; Johnstone, E.C.; Marsh, L. Postmortem evidence of structural brain changes in schizophrenia. Differences in brain weight, temporal horn area, and parahippocampal gyrus compared with affective disorder. Arch. Gen. Psychiatry 1986, 43, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Pakkenberg, B. Post-mortem study of chronic schizophrenic brains. Br. J. Psychiatry 1987, 151, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, S.; Kim, J.J.; Potkin, S.G.; Hetrick, W.P.; Bunney, W.E., Jr.; Jones, E.G. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch. Gen. Psychiatry 1996, 53, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Stewart, D.G.; Friedman, J.I.; Buchsbaum, M.; Harvey, P.D.; Hof, P.R.; Buxbaum, J.; Haroutunian, V. White matter changes in schizophrenia: Evidence for myelin-related dysfunction. Arch. Gen. Psychiatry 2003, 60, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, A.; Conn, P.J. The hippocampo-prefrontal pathway: A possible therapeutic target for negative and cognitive symptoms of schizophrenia. Future Neurol. 2015, 10, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F.; Kern, R.S.; Heaton, R.K. Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophr. Res. 2004, 72, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.; Richter-Landsberg, C.; Leibfritz, D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev. Neurosci. 1993, 15, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Bustillo, J.R. Use of proton magnetic resonance spectroscopy in the treatment of psychiatric disorders: A critical update. Dialogues Clin. Neurosci. 2013, 15, 329–337. [Google Scholar] [PubMed]
- Rae, C.D. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res. 2014, 39, 1–36. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzelecki, D.; Podgórski, M.; Kałużyńska, O.; Gawlik-Kotelnicka, O.; Stefańczyk, L.; Kotlicka-Antczak, M.; Gmitrowicz, A.; Grzelak, P. Supplementation of Antipsychotic Treatment with the Amino Acid Sarcosine Influences Proton Magnetic Resonance Spectroscopy Parameters in Left Frontal White Matter in Patients with Schizophrenia. Nutrients 2015, 7, 8767-8782. https://0-doi-org.brum.beds.ac.uk/10.3390/nu7105427
Strzelecki D, Podgórski M, Kałużyńska O, Gawlik-Kotelnicka O, Stefańczyk L, Kotlicka-Antczak M, Gmitrowicz A, Grzelak P. Supplementation of Antipsychotic Treatment with the Amino Acid Sarcosine Influences Proton Magnetic Resonance Spectroscopy Parameters in Left Frontal White Matter in Patients with Schizophrenia. Nutrients. 2015; 7(10):8767-8782. https://0-doi-org.brum.beds.ac.uk/10.3390/nu7105427
Chicago/Turabian StyleStrzelecki, Dominik, Michał Podgórski, Olga Kałużyńska, Oliwia Gawlik-Kotelnicka, Ludomir Stefańczyk, Magdalena Kotlicka-Antczak, Agnieszka Gmitrowicz, and Piotr Grzelak. 2015. "Supplementation of Antipsychotic Treatment with the Amino Acid Sarcosine Influences Proton Magnetic Resonance Spectroscopy Parameters in Left Frontal White Matter in Patients with Schizophrenia" Nutrients 7, no. 10: 8767-8782. https://0-doi-org.brum.beds.ac.uk/10.3390/nu7105427