Antioxidant and Antihypertensive Effects of a Chemically Defined Fraction of Syrah Red Wine on Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Wine Sample and Fractionation
2.3. Total Phenolic Content
2.4. Antioxidant Assays
2.5. Quantification of Trans-Resveratrol and Quercetin by HPLC-UV Analysis
2.6. Chemical Characterization by Liquid Chromatography Coupled to Mass Spectrometry (LC–MS) Analysis
2.7. Animals and Treatment
2.8. Blood Pressure and Heart Rate Recordings
2.9. Tiobarbituric Acid Reactive Species (TBARS) Assay
2.10. Vascular Reactivity Studies in Isolated Rat Superior Mesenteric Artery Rings
2.11. Statistical Analysis
3. Results
3.1. Phenolic Content and Antioxidant Activity
3.2. Chemical Characterization of Fr 2 SySFV by UPLC-ESI-QTOF-HRMS
3.3. Treatment with Fr 2 SySFV Reduces Blood Pressure in Spontaneoulsy Hypertensive Rats (SHR) In Vivo
3.4. Treatment with Fr 2 SySFV Reduces Oxidative Stress in Spontaneoulsy Hypertensive Rats
3.5. Fr 2 Sy SFV Induces Endothelium-Independent Vasorelaxation in Isolated Rat Superior Mesenteric Rings
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [PubMed]
- Padilla, E.; Ruiz, E.; Redondo, S.; Gordillo-Moscoso, A.; Slowing, K.; Tejerina, T. Relationship between vasodilation capacity and phenolic content of Spanish wines. Eur. J. Pharmacol. 2005, 517, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.d.S.; Silani, I.d.S.V.; Toaldo, I.M.; Corrêa, L.C.; Biasoto, A.C.T.; Pereira, G.E.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the northeast region of Brazil. Food Chem. 2014, 161, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lucena, A.P.S.; Nascimento, R.J.B.; Maciel, J.A.C.; Tavares, J.X.; Barbosa-Filho, J.M.; Oliveira, E.J. Antioxidant activity and phenolics content of selected Brazilian wines. J. Food Comp. Anal. 2010, 23, 30–36. [Google Scholar] [CrossRef]
- Botelho-Ono, M.S.; Pina, H.V.; Sousa, K.H.F.; Nunes, F.C.; Medeiros, I.A.; Braga, V.A. Acute superoxide scavenging restores depressed baroreflex sensitivity in renovascular hypertensive rats. Auton. Neurosci. 2011, 159, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Braga, V.A.; Medeiros, I.A.; Ribeiro, T.P.; França-Silva, M.S.; Botelho-Ono, M.S.; Guimarães, D.D. Angiotensin-ii-induced reactive oxygen species along the sfo-pvn-rvlm pathway: Implications in neurogenic hypertension. Braz. J. Med. Biol. Res. 2011, 44, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Porpino, S.K.P.; Zollbrecht, C.; Peleli, M.; Montenegro, M.F.; Brandão, M.C.R.; Athayde-Filho, P.F.; França-Silva, M.S.; Larsson, E.; Lundberg, J.O.; Weitzberg, E.; et al. Nitric oxide generation by the organic nitrate ndbp attenuates oxidative stress and angiotensin ii-mediated hypertension: Organic nitrate and hypertension. Br. J. Pharmacol. 2016, 173, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Lazartigues, E. Inflammation and neurogenic hypertension: A new role for the circumventricular organs? Circ. Res. 2010, 107, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Pedro-Botet, J.; Covas, M.I.; Martín, S.; Rubiés-Prat, J. Decreased endogenous antioxidant enzymatic status in essential hypertension. J. Hum. Hypertens. 2000, 14, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.C.; Reckelhoff, J.F. Role of angiotensin and oxidative stress in essential hypertension. Hypertension 1999, 34, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.C.; Lazartigues, E.; Sharma, R.V.; Davisson, R.L. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ. Res. 2004, 95, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Touyz, R.M. Nadph oxidases, reactive oxygen species, and hypertension: Clinical implications and therapeutic possibilities. Diabetes Care 2008, 31, S170–S180. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, V.; Jain, S. Effect of garlic supplementation on oxidized low density lipoproteins and lipid peroxidation in patients of essential hypertension. Mol. Cell. Biochem. 2004, 266, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [PubMed]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Micek, A.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the hapiee study. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Medina-Remon, A.; Zamora-Ros, R.; Rotches-Ribalta, M.; Andres-Lacueva, C.; Martinez-Gonzalez, M.A.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; et al. Total polyphenol excretion and blood pressure in subjects at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Association between polyphenol intake and hypertension in adults and older adults: A population-based study in Brazil. PLoS ONE 2016, 11, e0165791. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; O’Reilly, E.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Lajous, M.; Rossignol, E.; Fagherazzi, G.; Perquier, F.; Scalbert, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C. Flavonoid intake and incident hypertension in women. Am. J. Clin. Nutr. 2016, 103, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.E.; Parker, C.; Li, L.; Perlman, J.A.; Frei, B.; Ivanov, V.; Deak, L.R.; Iafrati, M.D.; Folts, J.D. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 2001, 103, 2792–2798. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, C.; Adamopoulos, K.; Lymperaki, E.; Iliadis, S.; Papapreponis, P.; Kourtidou-Papadeli, C. Cardiovascular risk and benefits from antioxidant dietary intervention with red wine in asymptomatic hypercholesterolemics. Clin. Nutr. ESPEN 2015, 10, e224–e233. [Google Scholar] [CrossRef] [PubMed]
- Luciano, M.N.; Ribeiro, T.P.; França-Silva, M.S.; do Nascimento, R.J.B.; de Jesus Oliveira, E.; França, K.C.; Antunes, A.A.; Nakao, L.S.; Aita, C.A.M.; Braga, V.A.; et al. Uncovering the vasorelaxant effect induced by vale do são francisco red wine: A role for nitric oxide. J. Cardiovasc. Pharmacol. 2011, 57, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, N.; Nardini, N.; Baldi, N.; Scaccini, N. Antioxidant activity of different phenolic fractions separated from an Italian red wine. J. Agric. Food Chem. 1998, 46, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of total phenolics. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., Sporns, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Garcez, F.R.; Garcez, W.S.; Hamerski, L.; Miguita, C.H. Fenilpropanóides e outros constituintes bioativos de nectandra megapotamica. Quím. Nova 2009, 32, 407–411. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Braga, V.A. Dietary salt enhances angiotensin-ii-induced superoxide formation in the rostral ventrolateral medulla. Auton. Neurosci. 2010, 155, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; França-Silva, M.; Alves, N.; Porpino, S.; Braga, V. Quercetin improves baroreflex sensitivity in spontaneously hypertensive rats. Molecules 2012, 17, 12997–13008. [Google Scholar] [CrossRef] [PubMed]
- Menkovic, N.; Zivkovic, J.; Savikin, K.; Godjevac, D.; Zdunic, G. Phenolic composition and free radical scavenging activity of wine produced from Serbian autochtonous grape variety prokupac: A model approach. J. Serb. Chem. Soc. 2014, 79, 11–24. [Google Scholar] [CrossRef]
- Chavarria, G.; Bergamaschi, H.; Silva, L.C.d.; Santos, H.P.d.; Mandelli, F.; Guerra, C.C.; Flores, C.A.; Tonietto, J. Relações hídricas, rendimento e compostos fenólicos de uvas cabernet sauvignon em três tipos de solo. Bragantia 2011, 70, 481–487. [Google Scholar] [CrossRef]
- Peterlunger, E.; Sivilotti, P.; Colussi, V. Water stress increased polyphenolic quality in ‘merlot’ grapes. Acta Hortic. 2005, 689, 293–300. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Villaño, D.; Garcı́a-Parrilla, M.C.; Troncoso, A.M. Antioxidant activity of wines and relation with their polyphenolic composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In vivo antioxidant activity of grape, pomace and wine from three red varieties grown in Argentina: Its relationship to phenolic profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Antioxidant activities of flavonoids as bioactive components of food. Biochem. Soc. Trans. 1996, 24, 790–795. [Google Scholar] [CrossRef] [PubMed]
- De Souza Dias, F.; Silva, M.F.; David, J.M. Determination of quercetin, gallic acid, resveratrol, catechin and malvidin in Brazilian wines elaborated in the vale do são francisco using liquid–liquid extraction assisted by ultrasound and GC-MS. Food Anal. Method 2013, 6, 963–968. [Google Scholar] [CrossRef]
- Dias, F.d.S.; David, J.M.; David, J.P. Determination of phenolic acids and quercetin in brazilian red wines from vale do são francisco region using liquid-liquid ultrasound-assisted extraction and HPLC-DAD-MS. J. Braz. Chem. Soc. 2015, 27. [Google Scholar] [CrossRef]
- Shalashvili, A.; Ugrekhelidze, D.; Mitaishvili, T.; Targamadze, I.; Zambakhidze, N. Phenolic compounds of wines from georgian autochthonous grapes, rkatsiteli and saperavi, prepared by georgian (kakhetian) technology. ResearchGate 2012, 6, 99–103. [Google Scholar]
- Goldberg, D.M.; Tsang, E.; Karumanchiri, A.; Soleas, G.J. Quercetin and p-coumaric acid concentrations in commercial wines. Am. J. Enol. Vitic. 1998, 49, 142–151. [Google Scholar]
- Price, S.F.; Breen, P.J.; Valladao, M.; Watson, B.T. Cluster sun exposure and quercetin in pinot noir grapes and wine. Am. J. Enol. Vitic. 1995, 46, 187–194. [Google Scholar]
- Careri, M.; Corradini, C.; Elviri, L.; Nicoletti, I.; Zagnoni, I. Direct HPLC analysis of quercetin and trans-resveratrol in red wine, grape, and winemaking byproducts. J. Agric. Food Chem. 2003, 51, 5226–5231. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventos, R.M.; Romero-Perez, A.I.; Waterhouse, A.L.; de la Torre-Boronat, M.C. Direct HPLC analysis of cis- and trans-resveratrol and piceid isomers in Spanish red vitis vinifera wines. J. Agric. Food Chem. 1995, 43, 281–283. [Google Scholar] [CrossRef]
- Montsko, G.; Nikfardjam, M.S.P.; Szabo, Z.; Boddi, K.; Lorand, T.; Ohmacht, R.; Mark, L. Determination of products derived from trans-resveratrol UV photoisomerisation by means of HPLC–APCI-MS. J. Photochem. Photobiol. A 2008, 196, 44–50. [Google Scholar] [CrossRef]
- Tříska, J.; Vrchotová, N.; Olejníčková, J.; Jílek, R.; Sotolář, R. Separation and identification of highly fluorescent compounds derived from trans-resveratrol in the leaves of vitis vinifera infected by plasmopara viticola. Molecules 2012, 17, 2773–2783. [Google Scholar] [CrossRef] [PubMed]
- Hu, M. Commentary: Bioavailability of flavonoids and polyphenols: Call to arms. Mol. Pharm. 2007, 4, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prahalathan, P.; Raja, B. Vanillic acid: A potential inhibitor of cardiac and aortic wall remodeling in l-name induced hypertension through upregulation of endothelial nitric oxide synthase. Environ. Toxicol. Pharmacol. 2014, 38, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Junior, L.d.G.; Monteiro, M.M.d.O.; Carvalho, A.D.S.; de Queiroz, T.M.; Braga, V.d.A. Oral supplementation with the rutin improves cardiovagal baroreflex sensitivity and vascular reactivity in hypertensive rats. Appl. Physiol. Nutr. Metab. 2013, 38, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Jiménez, R.; Hurtado, B.; Moreno, J.M.; Rodríguez-Gómez, I.; López-Sepúlveda, R.; Zarzuelo, A.; Pérez-Vizcaino, F.; Tamargo, J.; Vargas, F.; et al. Lack of beneficial metabolic effects of quercetin in adult spontaneously hypertensive rats. Eur. J. Pharmacol. 2010, 627, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.P.; Oliveira, A.C.; Mendes-Junior, L.G.; França, K.C.; Nakao, L.S.; Schini-Kerth, V.B.; Medeiros, I.A. Cardiovascular effects induced by northeastern Brazilian red wine: Role of nitric oxide and redox sensitive pathways. J. Funct. Foods 2016, 22, 82–92. [Google Scholar] [CrossRef]
- Dharmashankar, K.; Widlansky, M.E. Vascular endothelial function and hypertension: Insights and directions. Curr. Hypertens. Rep. 2010, 12, 448–455. [Google Scholar] [CrossRef] [PubMed]
- De Moura, R.S.; Miranda, D.Z.; Pinto, A.C.A.; Sicca, R.F.; Souza, M.A.V.; Rubenich, L.M.S.; Carvalho, L.C.R.M.; Rangel, B.M.; Tano, T.; Madeira, S.V.F.; et al. Mechanism of the endothelium-dependent vasodilation and the antihypertensive effect of Brazilian red wine. J. Cardiovasc. Pharmacol. 2004, 44, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Diebolt, M.; Bucher, B.; Andriantsitohaina, R. Wine polyphenols decrease blood pressure, improve no vasodilatation, and induce gene expression. Hypertension 2001, 38, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Porteri, E.; Rizzoni, D.; De Ciuceis, C.; Boari, G.E.M.; Platto, C.; Pilu, A.; Miclini, M.; Agabiti Rosei, C.; Bulgari, G.; Agabiti Rosei, E. Vasodilator effects of red wines in subcutaneous small resistance artery of patients with essential hypertension. Am. J. Hypertens. 2010, 23, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, A.; Gojkovic-Bukarica, L.; Peric, M.; Nezic, D.; Djukanovic, B.; Markovic-Lipkovski, J.; Heinle, H. The mechanism of endothelium-independent relaxation induced by the wine polyphenol resveratrol in human internal mammary artery. J. Pharmacol. Sci. 2006, 101, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Rendig, S.V.; Symons, J.D.; Longhurst, J.C.; Amsterdam, E.A. Effects of red wine, alcohol, and quercetin on coronary resistance and conductance arteries. J. Cardiovasc. Pharmacol. 2001, 38, 219–227. [Google Scholar] [CrossRef] [PubMed]




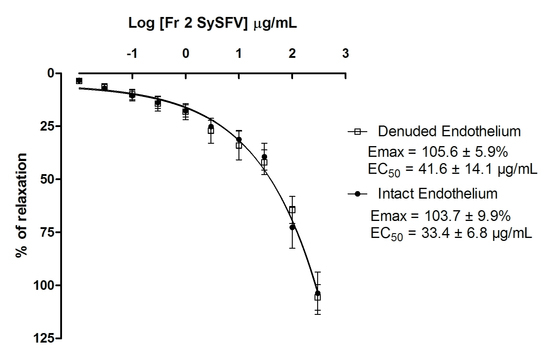
 ) and absence (
) and absence (  ) of functional endothelium. Results are expressed as mean ± SEM; n = 7 for rings with intact endothelium and n = 8 for rings with denuded endothelium.
) of functional endothelium. Results are expressed as mean ± SEM; n = 7 for rings with intact endothelium and n = 8 for rings with denuded endothelium.
 ) and absence (
) and absence (  ) of functional endothelium. Results are expressed as mean ± SEM; n = 7 for rings with intact endothelium and n = 8 for rings with denuded endothelium.
) of functional endothelium. Results are expressed as mean ± SEM; n = 7 for rings with intact endothelium and n = 8 for rings with denuded endothelium.
| Samples | Total Phenolic Content (mg GAE/100 mg) | EC50 (µg/mL) | |
|---|---|---|---|
| DPPH | ABTS | ||
| Fr 1 SySFV | 5.57 ± 0.01 | 56.27 ± 5.50 | 90.48 ± 1.34 |
| Fr 2 SySFV | 58.45 ± 0.01 | 3.4 ± 0.03 | 4.65 ± 0.04 |
| Fr 3 SySFV | 26.29 ± 0.03 | 13.25 ± 0.07 | 11.47 ± 0.55 |
| Ascorbic acid | - | 4.38 ± 0.07 | - |
| Trolox | - | - | 3.77 ± 0.02 |
| * Peak | Retention Time (min) | λmax | Compounds | Molecular Formula | [M−H]− | Fragments (m/z) | Calc. Mass | Error (ppm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.15 | 278 | Catechin | C15H14O6 | 289.0706 | 245.0816 | 289.0712 | 2.10 |
| 2 | 4.48 | 278 | Procyanidin dimer a | C30H26O12 | 577.1352 | 407.0798, 305.0674 | 577.1352 | 1.04 |
| 3 | 4.48 | 278 | Procyanidin dimer a | C30H26O12 | 577.1331 | 407.0758, 289.0720 | 577.1352 | 2.60 |
| 4 | 4.50 | 322 | Caffeic acid | C9H8O4 | 179.0346 | 160.8423, 135.0452 | 179.0344 | 1.11 |
| 5 | 4.71 | 278 | Epicatechin | C15H14O6 | 289.0710 | 245.0824 | 289.0712 | 0.72 |
| 6 | 4.98 | 282 | Procyanidin dimer monoglycoside | C36H36O17 | 739.1848 | 577.1340, 455.1034 | 739.1879 | >10 |
| 7 | 5.14 | 285 | Myricetin hexoside | C21H20O13 | 479.0822 | 316.0234 | 479.0825 | 0.63 |
| 8 | 5.21 | 285 | Dihydroquercetin hexoside | C21H22O12 | 465.1012 | 319.0827, 301.0351 | 465.1033 | 4.51 |
| 9 | 5.31 | 308 | p-Coumaric acid | C9H8O3 | 163.0399 | 119.0505 | 163.0395 | 2.45 |
| 10 | 5.56 | 272 | Syringic acid | C9H10O5 | 197.0453 | 160.8495 | 197.0450 | 1.52 |
| 11 | 5.56 | 374 | Myricetin | C15H10O8 | 317.0301 | 259.0278 | 317.0303 | 1.26 |
| 12 | 5.70 | ND | Epigallocatechin-coumaroyl a | C24H20O9 | 451.1026 | 341.0581, 255.8171 | 451.1035 | 0.66 |
| 13 | 5.71 | 357 | Quercertin hexoside | C21H20O12 | 463.0852 | 300.0280, 271.0253 | 463.0876 | 5.20 |
| 14 | 5.73 | 357 | Myricetin methyl ether hexoside | C22H22O13 | 493.0988 | 449.1082, 333.0980 | 493.0988 | 1.22 |
| 15 | 5.75 | 286 | Dihydrokaempferol hexoside | C21H22O11 | 449.1085 | 285.0404, 229.1086 | 449.1085 | 0.22 |
| 16 | 6.29 | 283 | Epigallocatechin-coumaroyl a | C24H20O9 | 451.1018 | 341.0667, 271.0651 | 451.1035 | 2.43 |
| 17 | 6.34 | 358 | Myricetin dimethyl ether hexoside | C23H24O13 | 507.1122 | 477.1027, 341.1033 | 417.1114 | 3.35 |
| 18 | 6.44 | 282 | Dihydrokaempferol-rhamnoside | C21H22O10 | 433.1139 | 353.1249, 267.1602 | 433.1140 | 0.92 |
| 19 | 6.90 | 305 | Epigallocatechin-cinnamoyl | C24H20O8 | 435.1063 | 341.0666, 285.0812 | 435.1085 | 3.90 |
| 20 | 7.63 | 371 | Quercetin | C15H10O7 | 301.0345 | 273.0420, 197.8082 | 301.0348 | 1.33 |
| 21 | 7.71 | 374 | Myricetin methyl ether | C16H12O8 | 331.0448 | 301.0353, 197.8083 | 331.0459 | 3.02 |
| 22 | 8.21 | 324 | Dimethoxy-cinnamic acid | C11H12O4 | 207.0671 | 161,0255, 130.0462 | 207.0663 | 8.24 |
| 23 | 8.61 | 360 | Luteolin | C15H10O6 | 285.0404 | 239.9008, 197.8085 | 285.0399 | 1.40 |
| 24 | 8.86 | 360 | Quercetin methyl ether | C16H12O7 | 315.0510 | 300.0280, 197.8084 | 315.0510 | 1.58 |
| 25 | 9.41 | 309 | Methyl methoxycinnamate | C11H12O3 | 191.0715 | 174.9576, 145.0302 | 191.0714 | 3.66 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, E.A.d.; Alves, N.F.B.; Monteiro, M.M.d.O.; Cavalcanti, C.d.O.; Silva, T.M.S.d.; Silva, T.M.G.d.; Braga, V.d.A.; Oliveira, E.d.J. Antioxidant and Antihypertensive Effects of a Chemically Defined Fraction of Syrah Red Wine on Spontaneously Hypertensive Rats. Nutrients 2017, 9, 574. https://0-doi-org.brum.beds.ac.uk/10.3390/nu9060574
Figueiredo EAd, Alves NFB, Monteiro MMdO, Cavalcanti CdO, Silva TMSd, Silva TMGd, Braga VdA, Oliveira EdJ. Antioxidant and Antihypertensive Effects of a Chemically Defined Fraction of Syrah Red Wine on Spontaneously Hypertensive Rats. Nutrients. 2017; 9(6):574. https://0-doi-org.brum.beds.ac.uk/10.3390/nu9060574
Chicago/Turabian StyleFigueiredo, Eugênia Abrantes de, Naiane Ferraz Bandeira Alves, Matheus Morais de Oliveira Monteiro, Clenia de Oliveira Cavalcanti, Tania Maria Sarmento da Silva, Telma Maria Guedes da Silva, Valdir de Andrade Braga, and Eduardo de Jesus Oliveira. 2017. "Antioxidant and Antihypertensive Effects of a Chemically Defined Fraction of Syrah Red Wine on Spontaneously Hypertensive Rats" Nutrients 9, no. 6: 574. https://0-doi-org.brum.beds.ac.uk/10.3390/nu9060574