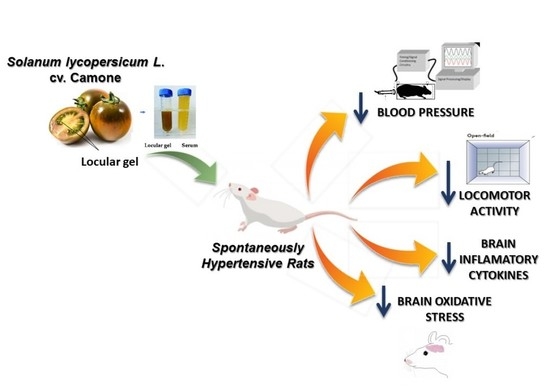
Effects of Aqueous Extract of Lycopersicum esculentum L. var. “Camone” Tomato on Blood Pressure, Behavior and Brain Susceptibility to Oxidative Stress in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Housing and Treatments
2.2.1. Blood Pressure Measurement
2.2.2. Open Field Test
2.2.3. Brain Cortical Slices
2.2.4. Brain IL-6, IL-1β and TNF-α Content
2.3. Statistical Analyses
3. Results
3.1. Effects of Tomato Gel/Serum, Tomatine and Captopril on Body Weight and Systolic Blood Pressure of SHRs and WKY Rats
3.2. Open Field Behaviors
3.3. Oxidative Damage and Inflammatory Cytokine Contents of Brain Slices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leone, G.; Consumi, M.; Franzi, C.; Tamasi, G.; Lamponi, S.; Donati, A.; Magnani, A.; Rossi, C.; Bonechi, C. Development of liposomal formulations to potentiate natural lovastatin inhibitory activity towards 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase. J. Drug Deliv. Sci. Tec. 2018, 43, 107–112. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef]
- Tamasi, G.; Pardini, A.; Bonechi, C.; Donati, A.; Pessina, F.; Marcolongo, P.; Gamberucci, A.; Leone, G.; Consumi, M.; Magnani, A.; et al. Characterization of nutraceutical components in tomato pulp, skin and locular gel. Eur. Food Res. Technol. 2019, 245, 907–918. [Google Scholar] [CrossRef]
- Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Bonechi, C.; Rossi, C.; Giunti, R.; Barone, V.; Borghini, A.; Fiorenzani, P.; et al. Chemical characterisation and antihypertensive effects of locular gel and serum of Lycopersicum esculentum L. var. “Camone” tomato in spontaneously hypertensive rats. Molecules 2020, 25, 3758. [Google Scholar] [CrossRef] [PubMed]
- Taveira, M.; Sousa, C.; Valentão, P.; Ferreres, F.; Teixeira, J.P.; Andrade, P.B. Neuroprotective effect of steroidal alkaloids on glutamate-induced toxicity by preserving mitochondrial membrane potential and reducing oxidative stress. J. Steroid Biochem. Mol. Biol. 2014, 140, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Bioactive Compounds of Tomatoes as Health Promoters. In Natural Bioactive Compounds from Fruits and Vegetables, 2nd ed.; Rodrigues da Silva, L., Silva, B.M., Eds.; Bentham Science Publishers: Sharjah, UAE, 2016; Chapter 3; pp. 48–91. [Google Scholar]
- Alissa, E.M.; Ferns, G.A. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J. Nutr. Metab. 2012, 2012, 569486. [Google Scholar] [CrossRef] [Green Version]
- Chiaino, E.; Micucci, M.; Cosconati, S.; Novellino, E.; Budriesi, R.; Chiarini, A.; Frosini, M. Olive leaves and hibiscus flowers extracts-based preparation protect brain from oxidative stress-induced injury. Antioxidant 2020, 9, 806. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W., Jr.; Materson, W.C.; Oparil, S.; Wright, J.T.; et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [Green Version]
- Hay, M.; Barnes, C.; Huentelman, M.; Brinton, R.; Ryan, L. Hypertension and age-related cognitive impairment: Common risk factors and a role for precision aging. Curr. Hypertens. Rep. 2020, 22, 80. [Google Scholar] [CrossRef]
- Iadecola, C.; Davisson, R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, T.C.; Adams, Z.H.; Baker, R.P.; Hope, K.A.; Paton, J.F.R.; Hart, E.C.; Nightingale, A.K. Investigation and treatment of high blood pressure in young people: Too much medicine or appropriate risk reduction? Hypertension 2020, 75, 6–22. [Google Scholar] [CrossRef]
- Adams, H.R.; Szilagyi, P.G.; Gebhardt, L.; Lande, M.B. Learning and attention problems among children with pediatric primary hypertension. Pediatrics 2010, 126, e1425-9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stobernack, T.; de Vries, S.P.W.; Rodrigues Pereira, R.; Pelsser, L.M.; Ter Braak, C.J.F.; Aarts, E.; van Baarlen, P.; Kleerebezem, M.; Frankena, K.; Hontelez, S. Biomarker research in ADHD: The impact of nutrition (brain)—Study protocol of an open-label trial to investigate the mechanisms underlying the effects of a few-foods diet on ADHD symptoms in children. BMJ Open 2019, 9, e029422. [Google Scholar] [CrossRef] [PubMed]
- Sagvolden, T.; Hendley, E.D.; Knardahl, S. Behavior of hypertensive and hyperactive rat strains: Hyperactivity is not unitarily determined. Physiol. Behav. 1992, 52, 49–57. [Google Scholar] [CrossRef]
- Okamoto, K.; Aoki, K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, A.M.; Ceccarelli, I.; Fiorenzani, P.; de Padova, A.M.; Massafra, C. Testosterone affects formalin-induced responses differently in male and female rats. Neurosci. Lett. 2004, 361, 262–264. [Google Scholar] [CrossRef]
- Pessina, F.; Frosini, M.; Marcolongo, P.; Fusi, F.; Saponara, S.; Gamberucci, A.; Valoti, M.; Giustarini, D.; Fiorenzani, P.; Gorelli, B. Antihypertensive, cardio- and neuro-protective effects of Tenebrio molitor (Coleoptera: Tenebrionidae) defatted larvae in spontaneously hypertensive rats. PLoS ONE 2020, 15, e0233788. [Google Scholar] [CrossRef]
- Coffman, T.M. Under pressure: The search for the essential mechanisms of hypertension. Nat. Med. 2011, 17, 1402–1409. [Google Scholar] [CrossRef]
- Pires, P.W.; Dams Ramos, C.M.; Matin, N.; Dorrance, A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1598–H1614. [Google Scholar] [CrossRef]
- Mayhan, W.G.; Faraci, F.M.; Heistad, D.D. Disruption of the blood-brain barrier in cerebrum and brain stem during acute hypertension. Am. J. Physiol. 1986, 251, H1171–H1175. [Google Scholar] [CrossRef]
- Jagla, F.; Pechanova, O. Age-related cognitive impairment as a sign of geriatric neurocardiovascular interactions: May polyphenols play a protective role? Oxid. Med. Cell Longev. 2015, 2015, 721514. [Google Scholar] [CrossRef] [Green Version]
- Kluknavsky, M.; Balis, P.; Puzserova, A.; Radosinska, J.; Berenyiova, A.; Drobna, M.; Lukac, S.; Muchova, J.; Bernatova, I. (-)-Epicatechin prevents blood pressure increase and reduces locomotor hyperactivity in young spontaneously hypertensive rats. Oxid Med. Cell Longev. 2016, 2016, 6949020. [Google Scholar] [CrossRef] [Green Version]
- Giardina, W.J.; Ebert, D.M. Positive effects of captopril in the behavioral despair swim test. Biol Psychiatry 1989, 25, 697–702. [Google Scholar] [CrossRef]
- Repova, K.; Aziriova, S.; Kovacova, D.; Trubacova, S.; Baka, T.; Kanska, R.; Barta, A.; Stanko, P.; Zorad, S.; Molcan, L.; et al. Lisinopril reverses behavioural alterations in spontaneously hypertensive rats. Gen. Physiol Biophys. 2019, 38, 265–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubcevic, J.; Watkins, J.; Perez, P.D.; Colon-Perez, L.M.; Long, M.T.; Febo, M.; Hayward, L. MEMRI reveals altered activity in brain regions associated with anxiety, locomotion, and cardiovascular reactivity on the elevated plus maze in the WKY vs. SHR rats. Brain Imaging Behav. 2018, 12, 1318–1331. [Google Scholar] [CrossRef]
- Lipska, B.K.; Weinberger, D.R. To model a psychiatric disorder in animals: Schizophrenia as a reality test. Neuropsychopharmacol. 2000, 23, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Calzavara, M.B.; Levin, R.; Medrano, W.A.; Almeida, V.; Sampaio, A.P.; Barone, L.C.; Frussa-Filho, R.; Abílio, V.C. Effects of antipsychotics and amphetamine on social behaviors in spontaneously hypertensive rats. Behav. Brain Res. 2011, 225, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.F.; Eufrásio, R.Á.; Gouvêa, D.A.; Diana, M.C.; Santos, C.M.; Swardfager, W.; Abílio, V.C.; Cogo-Moreira, H. A schizophrenia-like behavioral trait in the SHR model: Applying confirmatory factor analysis as a new statistical tool. Prog. Neuropsychopharmacol Biol. Psychiatry 2018, 85, 16–22. [Google Scholar] [CrossRef]
- Bruno, R.M.; Ghiadoni, L. Polyphenols, antioxidants and the sympathetic nervous system. Curr. Pharm. Des. 2018, 24, 130–139. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Neuroinflammation and sympathetic overactivity: Mechanisms and implications in hypertension. Auton. Neurosci. 2018, 210, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Winklewski, P.J.; Radkowski, M.; Wszedybyl-Winklewska, M.; Demkow, U. Brain inflammation and hypertension: The chicken or the egg? J. Neuroinflammation 2015, 12, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Liu, J.J.; Cui, W.; Shi, X.L.; Guo, J.; Li, H.B.; Huo, C.J.; Miao, Y.M.; Zhang, M.; Yang, Q.; et al. Alpha lipoic acid supplementation attenuates reactive oxygen species in hypothalamic paraventricular nucleus and sympathoexcitation in high salt-induced hypertension. Toxicol. Lett. 2016, 241, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gao, Y.H.; Tian, D.K.; Zheng, J.P.; Zhu, C.Y.; Ke, Y.; Bian, K. Inflammation of different tissues in spontaneously hypertensive rats. Sheng Li Xue Bao 2006, 58, 318–323. [Google Scholar]
- Masson, G.S.; Costa, T.S.; Yshii, L.; Fernandes, D.C.; Soares, P.P.; Laurindo, F.R.; Scavone, C.; Michelini, L.C. Time-dependent effects of training on cardiovascular control in spontaneously hypertensive rats: Role for brain oxidative stress and inflammation and baroreflex sensitivity. PLoS ONE 2014, 9, e94927. [Google Scholar] [CrossRef] [PubMed]
- Loperena, R.; Harrison, D.G. Oxidative stress and hypertensive diseases. Med. Clin. North. Am. 2017, 101, 169–193. [Google Scholar] [CrossRef] [Green Version]
- Reitz, C.; Luchsinger, J.A. Relation of blood pressure to cognitive impairment and dementia. Curr. Hypertens. Rev. 2007, 3, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zaitone, S.A.; Ahmed, E.; Elsherbiny, N.M.; Mehanna, E.T.; El-Kherbetawy, M.K.; ElSayed, M.H.; Alshareef, D.M.; Moustafa, Y.M. Caffeic acid improves locomotor activity and lessens inflammatory burden in a mouse model of rotenone-induced nigral neurodegeneration: Relevance to Parkinson’s disease therapy. Pharmacol. Rep. 2019, 71, 32–41. [Google Scholar] [CrossRef]
- Huang, S.L.; He, H.B.; Zou, K.; Bai, C.H.; Xue, Y.H.; Wang, J.Z.; Chen, J.F. Protective effect of tomatine against hydrogen peroxide-induced neurotoxicity in neuroblastoma (SH-SY5Y) cells. J. Pharm. Pharmacol. 2014, 66, 844–854. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol microbial metabolites exhibit gut and blood-brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Inhibitors of Acetylcholinesterase and Butyrylcholinesterase Meet Immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omo-bowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and ar-ginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Huang, W.Y.; Fu, L.; Li, C.Y.; Xu, L.P.; Zhang, L.X.; Zhang, W.M. Quercetin, hyperin, and chlorogenic acid improve endothelial function by antioxidant, antiinflammatory, and ACE inhibitory effects. J. Food Sci. 2017, 82, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Uddin, M.M.; Dizdarevic, L.L.; Jørgensen, A.; Duttaroy, A.K. Inhibition of angiotensin-converting enzyme by aqueous extract of tomato. Eur. J. Nutr. 2014, 53, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Davidge, S.T.; Wu, J. Bioactive natural constituents from food sources-potential use in hypertension prevention and treatment. Crit. Rev. Food Sci. Nutr. 2013, 53, 615–630. [Google Scholar] [CrossRef] [PubMed]





| Diet | WKY Weight Gain (g) | SHR Weight Gain (g) |
|---|---|---|
| Vehicle | 41.0 ± 0.6 | 43.0 ± 8.0 |
| Captopril | 36.6 ± 2.3 | 38.0 ± 4.4 |
| Gel/serum | 39.5 ± 4.3 | 42.7 ± 3.6 |
| Tomatine | 39.5 ± 4.5 | 41.0 ± 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frosini, M.; Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Giunti, R.; Fiorenzani, P.; Aloisi, A.M.; Rossi, C.; Pessina, F. Effects of Aqueous Extract of Lycopersicum esculentum L. var. “Camone” Tomato on Blood Pressure, Behavior and Brain Susceptibility to Oxidative Stress in Spontaneously Hypertensive Rats. Pathophysiology 2021, 28, 189-201. https://0-doi-org.brum.beds.ac.uk/10.3390/pathophysiology28010012
Frosini M, Marcolongo P, Gamberucci A, Tamasi G, Pardini A, Giunti R, Fiorenzani P, Aloisi AM, Rossi C, Pessina F. Effects of Aqueous Extract of Lycopersicum esculentum L. var. “Camone” Tomato on Blood Pressure, Behavior and Brain Susceptibility to Oxidative Stress in Spontaneously Hypertensive Rats. Pathophysiology. 2021; 28(1):189-201. https://0-doi-org.brum.beds.ac.uk/10.3390/pathophysiology28010012
Chicago/Turabian StyleFrosini, Maria, Paola Marcolongo, Alessandra Gamberucci, Gabriella Tamasi, Alessio Pardini, Roberta Giunti, Paolo Fiorenzani, Anna Maria Aloisi, Claudio Rossi, and Federica Pessina. 2021. "Effects of Aqueous Extract of Lycopersicum esculentum L. var. “Camone” Tomato on Blood Pressure, Behavior and Brain Susceptibility to Oxidative Stress in Spontaneously Hypertensive Rats" Pathophysiology 28, no. 1: 189-201. https://0-doi-org.brum.beds.ac.uk/10.3390/pathophysiology28010012