Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity
Abstract
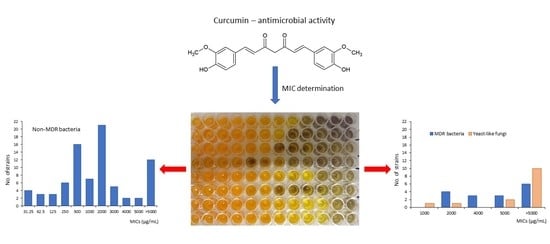
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Curcumin
4.2. Microbial Strains and Culture Media
4.3. Antimicrobial Activity
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [Green Version]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 263–288. [Google Scholar]
- Nair, K.P. Turmeric (Curcuma longa L.) and Ginger (Zingiber officinale Rosc.)—World’s Invaluable Medicinal Spices. The Agronomy and Economy of Turmeric and Ginger, 1st ed.; Springer Nature: Cham, Switzerland, 2019; pp. 1–243. [Google Scholar]
- Kwiecien, S.; Magierowski, M.; Majka, J.; Ptak-Belowska, A.; Wojcik, D.; Sliwowski, Z.; Magierowska, K.; Brzozowski, T. Curcumin: A potent protectant against esophageal and gastric disorders. Int. J. Mol. Sci. 2019, 20, 1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.T.; Lu, C.C.; Yen, G.C. Phytochemicals enhance antioxidant enzyme expression to protect against NSAID-induced oxidative damage of the gastrointestinal mucosa. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Singh, D.P.; Borse, S.P.; Rana, R.; Nivsarkar, M. Curcumin, a component of turmeric, efficiently prevents diclofenac sodium-induced gastroenteropathic damage in rats: A step towards translational medicine. Food Chem. Toxicol. 2017, 108, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and curcumin-like molecules: From spice to drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective properties of the golden spice curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Anasane, N.; Santos, C.A.D. Curcumin and curcumin-loaded nanoparticles: Antipathogenic and antiparasitic activities. Expert. Rev. Anti-Infect. Ther. 2020, 18, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azhdari, M.; Karandish, M.; Mansoori, A. Metabolic benefits of curcumin supplementation in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1289–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in health and diseases: Alzheimer’s disease and curcumin analogues, derivatives, and hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lu, J.; Jiang, B.; Guo, J. The roles of curcumin in regulating the tumor immunosuppressive microenvironment (Review). Oncol. Lett. 2020, 19, 3059–3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schraufstätter, E.; Bernt, H. Antibacterial action of curcumin and related compounds. Nature 1949, 164, 456–457. [Google Scholar] [CrossRef]
- Lutomski, J.; Kędzia, B.; Dębska, W. Wirkung des Äthanolextraktes und aktiver Substanzen aus Curcuma longa auf Bakterien und Pilze (Effect of the ethanol extract and active substances from Curcuma longa on bacteria and fungi). Planta Med. 1974, 26, 9–19. [Google Scholar] [CrossRef]
- Loo, C.Y.; Rohanizadeh, R.; Young, P.M.; Traini, D.; Cavaliere, R.; Whitchurch, C.B.; Lee, W.H. Combination of silver nanoparticles and curcumin nanoparticles for enhanced anti-biofilm activities. J. Agric. Food Chem. 2016, 64, 2513–2522. [Google Scholar] [CrossRef]
- Shukla, A.; Parmar, P.; Rao, P.; Goswami, D.; Saraf, M. Twin peaks: Presenting the antagonistic molecular interplay of curcumin with LasR and LuxR quorum sensing pathways. Curr. Microbiol. 2020. [Google Scholar] [CrossRef]
- Abdulrahman, H.; Misba, L.; Ahmad, S.; Khan, A.U. Curcumin induced photodynamic therapy mediated suppression of quorum sensing pathway of Pseudomonas aeruginosa: An approach to inhibit biofilm in vitro. Photodiagn. Photodyn. Ther. 2020, 30, 101645. [Google Scholar] [CrossRef]
- Packiavathy, I.A.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef]
- Das, P.; Gupta, G.; Velu, V.; Awasthi, R.; Dua, K.; Malipeddi, H. Formation of struvite urinary stones and approaches towards the inhibition—A review. Biomed. Pharmacother. 2017, 96, 361–370. [Google Scholar] [CrossRef]
- Teow, S.Y.; Liew, K.; Ali, S.A.; Khoo, A.S.B.; Peh, S.C. Antibacterial action of curcumin against Staphylococcus aureus: A brief review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef] [Green Version]
- Bahari, S.; Zeighami, H.; Mirshahabi, H.; Roudashti, S.; Haghi, F. Inhibition of Pseudomonas aeruginosa quorum sensing by subinhibitory concentrations of curcumin with gentamicin and azithromycin. J. Glob. Antimicrob. Resist. 2017, 10, 21–28. [Google Scholar] [CrossRef]
- Rangel-Castañeda, I.A.; Cruz-Lozano, J.R.; Zermeño-Ruiz, M.; Cortes-Zarate, R.; Hernández-Hernández, L.; Tapia-Pastrana, G.; Castillo-Romero, A. Drug susceptibility testing and synergistic antibacterial activity of curcumin with antibiotics against enterotoxigenic Escherichia coli. Antibiotics 2019, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Manoharlal, R.; Negi, A.S.; Prasad, R. Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res. 2010, 10, 570–578. [Google Scholar] [CrossRef]
- Lawhavinit, O.; Kongkathip, N.; Kongkathip, B. Antimicrobial activity of curcuminoids from Curcuma longa L. on pathogenic bacteria of shrimp and chicken. Kasetsart J. Nat. Sci. 2010, 44, 364–371. [Google Scholar]
- Betts, J.W.; Sharili, A.S.; La Ragione, R.M.; Wareham, D.W. In vitro antibacterial activity of curcumin-polymyxin B combinations against multidrug-resistant bacteria associated with traumatic wound infections. J. Nat. Prod. 2016, 79, 1702–1706. [Google Scholar] [CrossRef]
- Sasidharan, N.K.; Sreekala, S.R.; Jacob, J.; Nambisan, B. In vitro synergistic effect of curcumin in combination with third generation cephalosporins against bacteria associated with infectious diarrhea. Biomed. Res. Int. 2014, 2014, 561456. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yan, M.; Ma, R.; Ma, S. Synthesis and antibacterial activity of novel 4-bromo-1H-indazole derivatives as FtsZ inhibitors. Arch. Pharm. Chem. Life Sci. 2015, 348, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Gunes, H.; Gulen, D.; Mutlu, R.; Gumus, A.; Tas, T.; Topkaya, A.E. Antibacterial effects of curcumin: An in vitro minimum inhibitory concentration study. Toxicol. Ind. Health 2016, 32, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.D.; Santos, P.D.F.; Palazzi, N.C.; Leimann, F.V.; Fuchs, R.H.B.; Bracht, L.; Gonçalves, O.H. Production and characterization of curcumin microcrystals and evaluation of the antimicrobial and sensory aspects in minimally processed carrots. Food Funct. 2017, 8, 1851–1858. [Google Scholar] [CrossRef]
- Khan, M.; Ali, M.; Shah, W.; Shah, A.; Yasinzai, M.M. Curcumin-loaded self-emulsifying drug delivery system (cu-SEDDS): A promising approach for the control of primary pathogen and secondary bacterial infections in cutaneous leishmaniasis. Appl. Microbiol. Biotechnol. 2019, 103, 7481–7490. [Google Scholar] [CrossRef]
- Polaquini, C.R.; Morão, L.G.; Nazaré, A.C.; Torrezan, G.S.; Dilarri, G.; Cavalca, L.B.; Campos, D.L.; Silva, I.C.; Pereira, J.A.; Scheffers, D.J.; et al. Antibacterial activity of 3,3′-dihydroxycurcumin (DHC) is associated with membrane perturbation. Bioorg. Chem. 2019, 90, 103031. [Google Scholar] [CrossRef]
- Srivastava, P.; Shukla, M.; Kaul, G.; Chopra, S.; Patra, A.K. Rationally designed curcumin based ruthenium(II) antimicrobials effective against drug-resistant Staphylococcus aureus. Dalton Trans. 2019, 48, 11822–11828. [Google Scholar] [CrossRef]
- Neelakantan, P.; Subbarao, C.; Sharma, S.; Subbarao, C.V.; Garcia-Godoy, F.; Gutmann, J.L. Effectiveness of curcumin against Enterococcus faecalis biofilm. Acta Odontol. Scand. 2013, 71, 1453–1457. [Google Scholar] [CrossRef]
- Marickar, R.F.; Geetha, R.V.; Neelakantan, P. Efficacy of contemporary and novel intracanal medicaments against Enterococcus faecalis. J. Clin. Pediatr. Dent. 2014, 39, 47–50. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Antibacterial activity of curcumin via apoptosis-like response in Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 5505–5514. [Google Scholar] [CrossRef]
- Raorane, C.J.; Lee, J.H.; Kim, Y.G.; Rajasekharan, S.K.; García-Contreras, R.; Lee, J. Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front. Microbiol. 2019, 10, 990. [Google Scholar] [CrossRef]
- Betts, J.W.; Wareham, D.W. In vitro activity of curcumin in combination with epigallocatechin gallate (EGCG) versus multidrug-resistant Acinetobacter baumannii. BMC Microbiol. 2014, 14, 172. [Google Scholar] [CrossRef] [Green Version]
- Tajbakhsh, S.; Mohammadi, K.; Deilami, I.; Zandi, K.; Fouladvand, M.; Ramedani, E.; Asayesh, G. Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr. J. Biotechnol. 2008, 7, 3832–3835. [Google Scholar]
- Sharifi, S.; Fathi, N.; Memar, M.Y.; Khatibi, S.M.H.; Khalilov, R.; Negahdari, R.; Vahed, S.Z.; Dizaj, S.M. Anti-microbial activity of curcumin nanoformulations: New trends and future perspectives. Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Modi, N.H.; Panda, D.; Roy, N. Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ—A structural insight to unveil antibacterial activity of curcumin. Eur. J. Med. Chem. 2010, 45, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Alalwan, H.; Rajendran, R.; Lappin, D.F.; Combet, E.; Shahzad, M.; Robertson, D.; Nile, C.J.; Williams, C.; Ramage, G. The anti-adhesive effect of curcumin on Candida albicans biofilms on denture materials. Front. Microbiol. 2017, 8, 659. [Google Scholar] [CrossRef]
- Sharma, G.; Raturi, K.; Dang, S.; Gupta, S.; Gabrani, R. Combinatorial antimicrobial effect of curcumin with selected phytochemicals on Staphylococcus epidermidis. J. Asian Nat. Prod. Res. 2014, 16, 535–541. [Google Scholar] [CrossRef]
- Li, X.; Yin, L.; Ramage, G.; Li, B.; Tao, Y.; Zhi, Q.; Lin, H.; Zhou, Y. Assessing the impact of curcumin on dual-species biofilms formed by Streptococcus mutans and Candida albicans. Microbiol. Open 2019, 8, e937. [Google Scholar] [CrossRef] [Green Version]
- Sivasothy, Y.; Sulaiman, S.F.; Ooi, K.L.; Ibrahim, H.; Awang, K. Antioxidant and antibacterial activities of flavonoids and curcuminoids from Zingiber spectabile Griff. Food Control. 2013, 30, 714–720. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altunatmaz, S.S.; Aksu, F.Y.; Issa, G.; Kahraman, B.B.; Altiner, D.D.; Buyukunal, S. Antimicrobial effects of curcumin against L. monocytogenes, S. aureus, S.Typhimurium and E. coli O157: H7 pathogens in minced meat. Vet. Med. 2016, 61, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Gómez, S.; Querol-García, J.; Sánchez-Barrón, G.; Subias, M.; González-Alsina, À.; Franco-Hidalgo, V.; Albertí, S.; de Córdoba, S.R.; Fernández, F.J.; Vega, M.C. The antimicrobials anacardic acid and curcumin are not-competitive inhibitors of Gram-positive bacterial pathogenic glyceraldehyde-3-phosphate dehydrogenase by a mechanism unrelated to human C5a anaphylatoxin binding. Front. Microbiol. 2019, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Joung, D.K.; Kim, Y.S.; Kang, O.H.; Kim, S.B.; Seo, Y.S.; Kim, Y.C.; Lee, D.S.; Shin, D.W.; Kweon, K.T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, T.; Bais, H.P. Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J. Agric. Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef]
- Pandey, A.; Gupta, R.K.; Bhargava, A.; Agrawal, B. Antibacterial activities of curcumin bioconjugates. Int. J. Pharmacol. 2011, 7, 874–879. [Google Scholar] [CrossRef] [Green Version]
- Shariati, A.; Asadian, E.; Fallah, F.; Azimi, T.; Hashemi, A.; Sharahi, J.Y.; Moghadam, M.T. Evaluation of Nano-curcumin effects on expression levels of virulence genes and biofilm production of multidrug-resistant Pseudomonas aeruginosa isolated from burn wound infection in Tehran, Iran. Infect. Drug Resist. 2019, 12, 2223–2235. [Google Scholar] [CrossRef] [Green Version]
- Prasad, E.; Hameeda, B.; Rao, A.B.; Reddy, G. Biotransformation of curcumin for improved biological activity and antiproliferative activity on acute HT-29 human cell lines. Indian J. Biotechnol. 2014, 13, 324–329. [Google Scholar]
- Manchanda, G.; Sodhi, R.K.; Jain, U.K.; Chandra, R.; Madan, J. Iodinated curcumin bearing dermal cream augmented drug delivery, antimicrobial and antioxidant activities. J. Microencapsul. 2018, 35, 49–61. [Google Scholar] [CrossRef]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin nanoparticles: Preparation, characterization, and antimicrobial study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- Narayanan, V.S.; Muddaiah, S.; Shashidara, R.; Sudheendra, U.S.; Deepthi, N.C.; Samaranayake, L. Variable antifungal activity of curcumin against planktonic and biofilm phase of different candida species. Indian J. Dent. Res. 2020, 31, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Neelofar, K.; Shreaz, S.; Rimple, B.; Muralidhar, S.; Nikhat, M.; Khan, L.A. Curcumin as a promising anticandidal of clinical interest. Can. J. Microbiol. 2011, 57, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EUCAST. MIC Determination of Non-Fastidious and Fastidious Organisms. Available online: http://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 23 September 2019).
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests. Approved Standard, 11th ed.; CLSI document M02-A11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]

| Microbial Strains | Minimum Inhibitory Concentration, Mean MIC Values (µg/mL) | Median |
|---|---|---|
| 1. Gram-positive bacteria | ||
| Streptococcus pyogenes (n = 6) 1 | 31.25 (4×) 2, 62.5, 125 | 31.25 |
| S. agalactiae (n = 6) | 2000 (3×), 3000 (2×), 4000 | 2500 |
| Staphylococcus aureus (n = 6) | 125, 250 (3×), 500 (2×) | 250 |
| S. aureus ATCC 29213 | 250 | |
| S. haemolyticus (n = 6) | 500 (6×) | 500 |
| S. epidermidis (n = 6) | 500 (2×), 1000 (3×), 2000 | 1000 |
| Enterococcus faecalis (n = 6) | 62.5, 500 (3×), 1000, 2000 | 500 |
| Methicillin-resistant S. aureus (n = 4) | 2000, 4000, >5000 (2×) | >4500 |
| Methicillin-resistant S. haemolyticus (n = 3) | 2000, >5000 (2×) | >5000 |
| Vancomycin-resistant E. faecalis (n = 1) | 5000 | |
| Median | 500 3 a, >5000 4 b | |
| 2. Gram-negative bacteria | ||
| Acinetobacter lwoffii (n = 3) | 125, 250 (2×) | 250 |
| A. baumannii (n = 3) | >5000 (3×) | >5000 |
| Escherichia coli (n = 6) | 500 (2×), 1000, 2000 (2×), 3000 | 1500 |
| E. coli ATCC 25922 | 2000 | |
| Klebsiella oxytoca (n = 6) | 500, 1000, 2000 (2×), >5000 (2×) | 2000 |
| K. pneumoniae (n = 6) | 2000 (5×), 3000 | 2000 |
| Pseudomonas aeruginosa (n = 6) | 62.5, 2000 (2×), 3000, 5000, >5000 | 2500 |
| P. aeruginosa ATCC 27853 (Boston 41501) | 5000 | |
| P. aeruginosa NCTC 6749 | >5000 | |
| Proteus mirabilis (n = 6) | 1000, 2000 (2×), 4000, >5000 (2×) | 3000 |
| Serratia marcescens (n = 4) | 2000 (2×), >5000 (2×) | >3500 |
| Stenotrophomonas maltophilia (n = 1) | >5000 | |
| ESβL-positive E. coli (n = 4) | 2000, 4000, 5000 (2×) | 4500 |
| ESβL-positive P. mirabilis (n = 4) | 2000, 4000, >5000 (2×) | >4500 |
| Median | 2000 b, >4500 b | |
| 3. Yeast-like fungi | ||
| Candida albicans (n = 10) | 1000, 2000, 5000, >5000 (7×) | >5000 |
| C. glabrata (n = 2) | >5000 (2×) | >5000 |
| C. tropicalis (n = 1) | >5000 | |
| Saccharomyces cerevisiae (n = 1) | 5000 | |
| Median | >5000 b | |
| Negative control | ||
| 15% DMSO | >5000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13070153
Adamczak A, Ożarowski M, Karpiński TM. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals. 2020; 13(7):153. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13070153
Chicago/Turabian StyleAdamczak, Artur, Marcin Ożarowski, and Tomasz M. Karpiński. 2020. "Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity" Pharmaceuticals 13, no. 7: 153. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13070153