Imidazole and Imidazolium Antibacterial Drugs Derived from Amino Acids
Abstract
:1. Introduction
2. Results
2.1. Synthesis
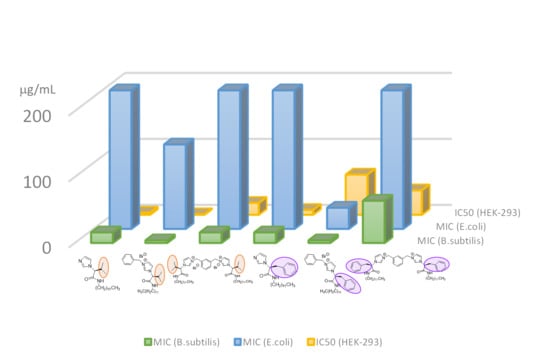
2.2. Antibacterial and Cytotoxicity Studies
2.3. Aggregation Studies
2.3.1. Fluorescence Spectroscopy
2.3.2. Optical Microscopy and Scanning Electron Microscopy (SEM)
2.3.3. UV-Vis Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Reagents and Culture Media
4.1.2. Synthesis and Characterization
4.1.3. Microorganisms and Growth Conditions
4.2. Methods
4.2.1. Bacterial Proliferation Assay in Presence of Imidazole Derivatives
4.2.2. Log P Calculation and Retention Time Determination
4.2.3. H NMR Studies
4.2.4. Fluorescence Spectroscopy Measurements
4.2.5. Optical Images
4.2.6. Scanning Electron Microscopy (SEM)
4.2.7. UV-Vis Spectroscopy
4.2.8. Cell Proliferation Assay for Cytotoxicity Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rani, N.; Sharma, A.; Singh, R. Imidazoles as Promising Scaffolds for Antibacterial Activity: A Review. Mini-Rev. Med. Chem. 2013, 13, 1812–1835. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.T.; Wang, Z.C.; Sang, Y.L.; Tao, X.X.; Zhu, H.L. Exploration of Structure-Based on Imidazole Core as Antibacterial Agents. Curr. Top. Med. Chem. 2013, 13, 3118–3130. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Li, Q.; Liu, D.; Ding, M.W. Synthesis, Fungicidal Activity, and Sterol 14α-Demethylase Binding Interaction of 2-Azolyl-3,4-dihydroquinazolines on Penicillium digitatum. J. Agric. Food Chem. 2013, 61, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, B.; Fan, Z.J.; Liu, X.M.; Wu, Q.F.; Li, H.P.; Wang, H.X. Synthesis of Novel 3,4-Chloroisothiazole-Based Imidazoles as Fungicides and Evaluation of Their Mode of Action. J. Agric. Food Chem. 2018, 66, 7319–7327. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.F.; Wu, X.H.; Tu, X.; Wang, G.X. Synthesis and biological evaluation of coumarin derivatives containing imidazole skeleton as potential antibacterial agents. Eur. J. Med. Chem. 2018, 143, 958–969. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Wang, M.-W.; Zeng, D.; Xiang, M.; Rao, J.-R.; Liu, Q.-Q.; Liu, L.-W.; Wu, Z.-B.; Li, Z.; Song, B.A.; et al. Rational Optimization and Action Mechanism of Novel Imidazole (or Imidazolium)-Labeled 1,3,4 Oxadiazole Thioethers as Promising Antibacterial Agents against Plant Bacterial Diseases. J. Agric. Food Chem. 2019, 67, 3535–3545. [Google Scholar] [CrossRef]
- Rossi, R.; Ciofalo, M. An Updated Review on the Synthesis and Antibacterial Activity of Molecular Hybrids and Conjugates Bearing Imidazole Moiety. Molecules 2020, 25, 5133. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Kelley, S.P.; Gurau, G.; Rogers, R.D. Chemistry: Develop ionic liquid drugs. Nature 2015, 528, 188–189. [Google Scholar] [CrossRef] [Green Version]
- Hauss, D. Oral lipid-based formulations. J. Adv. Drug Deliv. Rev. 2007, 59, 667–676. [Google Scholar] [CrossRef]
- Guillory, J.K. Pharmaceutical Salts: Properties, Selection, and Use, 2nd ed.; Stahl, P.H., Wermuth, C.G., Eds.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef] [Green Version]
- Stoimenovski, J.; MacFarlane, D.R.; Bica, K.; Rogers, R.D. Crystalline vs. Ionic Liquid Salt Forms of Active Pharmaceutical Ingredients: A Position Paper. Pharm. Res. 2010, 27, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Shadid, M.; Gurau, G.; Shamshina, J.L.; Chuang, B.-C.; Hailu, S.; Guan, E.; Chowdhury, S.K.; Wu, J.T.; Rizvi, S.A.A.; Griffin, R.J.; et al. Sulfasalazine in ionic liquid form with improved solubility and exposure. Med. Chem. Comm. 2015, 6, 1837–1841. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Prudencio, C.; Noronha, J.P.; Petrovski, Z. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef]
- Miskiewicz, A.; Ceranowicz, P.; Szymczak, M.; Bartus, K.; Kowalczyk, P. The Use of Liquids Ionic Fluids as Pharmaceutically Active Substances Helpful in Combating Nosocomial Infections Induced by Klebsiella Pneumoniae New Delhi Strain, Acinetobacter Baumannii and Enterococcus Species. Int. J. Mol. Sci. 2018, 19, 2779. [Google Scholar] [CrossRef] [Green Version]
- Cuervo-Rodríguez, R.; Muñoz-Bonilla, A.; López-Fabal, F.; Fernández-García, M. Hemolytic and Antimicrobial Activities of a Series of Cationic Amphiphilic Copolymers Comprised of Same Centered Comonomers with Thiazole Moieties and Polyethylene Glycol Derivatives. Polymers 2020, 12, 972. [Google Scholar] [CrossRef]
- Messali, M.; Moussa, Z.; Alzahrani, A.Y.; El-Naggar, M.Y.; ElDouhaibi, A.S.; Judeh, Z.M.A.; Hammouti, B. Synthesis, characterization and the antimicrobial activity of new eco-friendly ionic liquids. Chemosphere 2013, 91, 1627–1634. [Google Scholar] [CrossRef]
- Wang, D.; Richter, C.; Rühling, A.; Drücker, P.; Siegmund, D.; Metzler-Nolte, N.; Glorius, F.; Galla, H.-J. A Remarkably Simple Class of Imidazolium-Based Lipids and Their Biological Properties. Chem. Eur. J. 2015, 21, 15123–15126. [Google Scholar] [CrossRef]
- Chen, H.-L.; Kao, H.-F.; Wang, J.-Y.; Wei, G.-T. Cytotoxicity of Imidazole Ionic Liquids in Human Lung Carcinoma A549 Cell Line. J. Chin. Chem. Soc. 2014, 61, 763–769. [Google Scholar] [CrossRef]
- Malhotra, S.V.; Kumar, V.; Velez, C.; Zayas, B. Imidazolium-Derived Ionic Salts Induce Inhibition of Cancerous Cell Growth through Apoptosis. MedChemComm 2014, 5, 1404–1409. [Google Scholar] [CrossRef]
- Pernak, J.; Sobaszkiewicz, K.; Mirska, I. Anti-microbial activities of ionic liquids. Green Chem. 2003, 5, 52–56. [Google Scholar] [CrossRef]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Nizameev, I.R.; Kadirov, M.K.; Kashapov, R.R.; Zakharova, Y.L. Supramolecular systems based on cationic imidazole-containing amphiphiles bearing hydroxyethyl fragment: Aggregation properties and functional activity. J. Mol. Liq. 2019, 289, 111058. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Perez, L.; Manresa, A. Micellization and antimicrobial properties of surface-active ionic liquids containing cleavable carbonate linkages. Langmuir 2017, 33, 6511–6520. [Google Scholar] [CrossRef] [Green Version]
- Gindri, I.M.; Siddiqui, D.A.; Bhardwaj, P.; Rodriguez, L.C.; Palmer, K.L.; Frizzo, C.P.; Martinsc, M.A.P.; Rodrigues, D.C. Dicationic imidazolium-based ionic liquids: A new strategy for non-toxic and antimicrobial materials. RSC Adv. 2014, 4, 62594–62602. [Google Scholar] [CrossRef]
- Pałkowski, Ł.; Błaszczyński, J.; Skrzypczak, A.; Błaszczak, J.; Kozakowska, K.; Wróblewska, J.; Kożuszko, S.; Gospodarek, E.; Krysiński, J.; Słowiński, R. Antimicrobial activity and SAR study of new gemini imidazolium-based chlorides. J. Chem. Biol. Drug Des. 2014, 83, 278–288. [Google Scholar] [CrossRef]
- Voloshina, A.D.; Gumerova, S.K.; Sapunova, A.S.; Kulik, N.V.; Mirgorodskaya, A.B.; Kotenko, A.A.; Prokopyeva, T.M.; Mikhailov, V.A.; Zakharova, L.Y.; Sinyashin, O.G. The structure—Activity correlation in the family of dicationic imidazolium surfactants: Antimicrobial properties and cytotoxic effect. BBA Gen. Subj. 2020, 1864, 129728. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qin, H.; Ding, L.; Huo, S.; Deng, Q.; Zhao, B.; Meng, L.; Yan, T. Preparation of a novel class of cationic gemini imidazolium surfactants containing amide groups as the spacer: Their surface properties and antimicrobial activity. J. Surfactant Deterg. 2014, 17, 1099–1106. [Google Scholar] [CrossRef]
- Kapitanov, I.V.; Jordan, A.; Karpichev, Y.; Spulak, M.; Perez, L.; Kellett, A.; Kümmerer, K.; Gathergood, N. Synthesis, self-assembly, bacterial and fungal toxicity, and preliminary biodegradation studies of a series of L-phenylalanine-derived surface-active ionic liquids. Green Chem. 2019, 21, 1777–1794. [Google Scholar] [CrossRef]
- González, L.; Escorihuela, J.; Altava, B.; Burguete, M.I.; Luis, S.V. Chiral Room Temperature Ionic Liquids as Enantioselective Promoters for the Asymmetric Aldol Reaction. Eur. J. Org. Chem. 2014, 2014, 5356–5363. [Google Scholar] [CrossRef]
- González, L.; Altava, B.; Bolte, M.; Burguete, M.I.; García-Verdugo, E.; Luis, S.V. Synthesis of Chiral Room Temperature Ionic Liquids from Amino Acids–Application in Chiral Molecular Recognition. Eur. J. Org. Chem. 2012, 2012, 4996–5009. [Google Scholar] [CrossRef] [Green Version]
- González-Mendoza, L.; Escorihuela, J.; Altava, B.; Burguete, M.I.; Luis, S.V. Application of optically active chiral bis(imidazolium) salts as potential receptors of chiral dicarboxylate salts of biological relevance. Org. Biomol. Chem. 2015, 13, 5450–5459. [Google Scholar] [CrossRef] [PubMed]
- González-Mendoza, L.; Escorihuela, J.; Altava, B.; Burguete, M.I.; Hernando, E.; Luis, S.V.; Quesada, R.; Vicent, C. Bis(imidazolium) salts derived from amino acids as receptors and transport agents for chloride anions. RSC Adv. 2015, 5, 34415–34423. [Google Scholar] [CrossRef] [Green Version]
- Baltazar, Q.Q.; Chandawalla, J.; Sawyer, K.; Anderson, J.L. Interfacial and micellar properties of imidazolium-based monocationic and dicationic ionic liquids. Colloids Surf. A 2007, 302, 150–156. [Google Scholar] [CrossRef]
- Kamboj, R.; Singh, S.; Bhadani, A.; Kataria, H.; Kaur, G. Gemini Imidazolium Surfactants: Synthesis and Their Biophysiochemical Study. Langmuir 2012, 28, 11969–11978. [Google Scholar] [CrossRef]
- Zhuang, L.-H. Synthesis and properties of novel ester-containing gemini imidazolium surfactants. J. Colloid Interface Sci. 2013, 408, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Bhadani, A.; Singh, T.M.S.; Sakai, K.; Sakai, H.; Abe, M. Structural diversity, physicochemical properties and application of imidazolium surfactants: Recent advances. Adv. Colloid Interface Sci. 2016, 231, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Galla, H.-J.; Drücker, P. Membrane interactions of ionic liquids and imidazolium salts. Biophys. Rev. 2018, 10, 735–746. [Google Scholar] [CrossRef]
- Knight, N.J.; Hernando, E.; Haynes, C.J.E.; Busschaert, M.; Clarke, H.J.; Takimoto, K.; García-Valverde, M.; Frey, J.G.; Quesada, R.; Gale, P.A. QSAR analysis of substituent effects on tambjamine anion transporters. Chem. Sci. 2016, 7, 1600–1608. [Google Scholar] [CrossRef] [Green Version]
- Gorczyca, M.; Korchowiec, B.; Korchowiec, J.; Trojan, S.; Rubio-Magnieto, J.; Luis, S.V.; Rogalska, E. A Study of the Interaction between a Family of Gemini Amphiphilic Pseudopeptides and Model Monomolecular Film Membranes Formed with a Cardiolipin. J. Phys. Chem. B 2015, 119, 6668–6679. [Google Scholar] [CrossRef]
- Barns, K.J.; Weisshaar, J.C. Single-cell, time-resolved study of the effects of the antimicrobial peptide alamethicin on Bacillus subtilis. Biochim. Biophys. Acta 2016, 1858, 725–732. [Google Scholar] [CrossRef]
- Ishiyama, A.; Otoguro, K.; Iwatsuki, M.; Namatame, M.; Nishihara, A.; Nonaka, K.; Kinoshita, Y.; Takahashi, Y.; Masuma, R.; Shiomi, K.; et al. In vitro and in vivo antitrypanosomal activities of three peptide antibiotics: Leucinostatin A and B, alamethicin I and tsushimycin. J. Antibiot. 2009, 62, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar Systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Photochemistry in Microheterogeneous Systems, 1st ed.; Academic Press: New York, NY, USA, 1987. [Google Scholar]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the determination of the critical micelle concentrationby the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frindi, M.; Michels, B.; Zana, R. Ultrasonic Absorption Studies of Surfactant Exchange between Micelles and Bulk Phase In Aqueous Micellar Solutions of Nonionic Surfactants with Short Alkyl Chains. 1,2-Hexanedlol and 1,2,3-Octanetrlol. J. Phys. Chem. 1991, 95, 4832–4837. [Google Scholar] [CrossRef]
- Regev, O.; Zana, R. Aggregation Behavior of Tyloxapol, a Nonionic Surfactant Oligomer, in Aqueous Solution. J. Colloid Interface Sci. 1999, 210, 8–17. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Goddard, E.D.; Turro, N.J.; Kuo, P.L. Fluorescence Probes for Critical Micelle Concentration. Langmuir 1985, 2, 352–355. [Google Scholar] [CrossRef]
- Liu, C.G.; Desai, K.G.H.; Chen, X.G.; Park, H.J. Linolenic acid-modified chitosan for formation of selfassembled nanoparticles. J. Agric. Food Chem. 2005, 53, 437–441. [Google Scholar] [CrossRef]
- Dong, X.; Liu, C. Preparation and Characterization of Self-Assembled Nanoparticles of Hyaluronic Acid-Deoxycholic Acid Conjugates. J. Nanomat. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Yoshimura, T.; Ichinokawa, T.; Kaji, M.; Esumi, K. Synthesis and surface-active properties of sulfobetaine-type zwitterionic gemini surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2006, 273, 208–212. [Google Scholar] [CrossRef]
- Gregory, J. Monitoring particle aggregation processes. Adv. Colloid Interface Sci. 2009, 147–148, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Luhrs, C.C.; Pérez-Luna, V.H. Controlled and Reversible Aggregation of Biotinylated Gold Nanoparticles with Streptavidin. J. Phys. Chem. B 2004, 108, 15631–15639. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bury-Moné, S. Antibacterial Therapeutic Agents: Antibiotics and Bacteriophages. In Reference Module in Biomedical Sciences, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–13. ISBN 9780128012383. [Google Scholar]
- Ghanema, O.B.; Mutaliba, M.J.A.; El-Harbawi, M.; Gonfaa, G.; Kait, C.F.; Alitheend, N.B.M.; Leveque, J.M. Effect of imidazolium-based ionic liquids on bacterial growth inhibition investigated via experimental and QSAR modelling studies. J. Hazard. Mater. 2015, 297, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.; Hansch, C.; Anderson, S. Structure-activity correlations for antibacterial agents on gram-positive and gram-negative cells. J. Med. Chem. 1968, 11, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.; Špulák, S.; Garcia, M.T.; Gathergood, N. Antimicrobial toxicity studies of ionic liquids leading to a ‘hit’ MRSA selective antibacterial imidazolium salt. Green Chem. 2012, 14, 1350–1356. [Google Scholar] [CrossRef]
- Roy, S.; Dey, J. Spontaneously Formed Vesicles of Sodium N-(11-Acrylamidoundecanoyl)-glycinate and l-Alaninate in Water. Langmuir 2005, 21, 10362–10369. [Google Scholar] [CrossRef]






| Entry | Compound | LogP a | Retention Time (min) b | E. coli | B. subtilis | HEK-293 IC50 μg/mL | ||
|---|---|---|---|---|---|---|---|---|
| MIC μg/mL e | MBC μg/mL e | MIC μg/mL e | MBC μg/mL e | |||||
| 1 | 1a | 3.01 c | 6.1 | >2000 | >2000 | 16 | 16 | 3.2 ± 0.5 |
| 2 | 1b | 3.63 d | 6.2 | 128 | 256 | 4 | 8 | 0.8 ± 0.2 |
| 3 | 1c | 4.83 d | 7.7 | 256 | 256 | 16 | 32 | 18 ± 4 |
| 4 | 2a | −0.67 c | 3.4 | >2000 | >2000 | >2000 | >2000 | >45 |
| 5 | 2b | −0.20 d | 3.7 | >2000 | >2000 | >1000 | >1000 | >79 |
| 6 | 2c | −1.34 d | 3.1 | 1000 | >2000 | 128 | 256 | >142 |
| 7 | 3a | 3.50 c | 6.7 | 1000 | >2000 | 16 | 16 | 7.3 ± 1.2 |
| 8 | 3b | 4.26 d | 8.8 | 32 | 128 | 4 | 4 | 61 ± 6 |
| 9 | 3c | 5.96 d | 10.0 | 2000 | 2000 | 64 | 64 | 37 ± 5 |
| 10 | Alamethicin [41] | -- | -- | 16 | -- | 62.5 f [42] | ||
| Entry | Amphiphilic Compound | CAC Fluorescence (mM) a | ||
|---|---|---|---|---|
| W b | CCM:W c | |||
| CAC1 | CAC2 | |||
| 1 | 1a | 0.085 | 0.004 | 0.045 |
| 2 | 1b | 0.084 | 0.006 | 0.048 |
| 3 | 1c | 0.010 | 0.016 | 0.21 |
| 4 | 2a | 4.31 | 2.89 | 5.4 |
| 5 | 2b | 4.57 | 3.46 | 8.4 |
| 6 | 2c | 2.34 | 2.25 | 3.54 |
| 7 | 3a | 0.033 | 0.018 | 0.325 |
| 8 | 3b | 0.098 | 0.063 | 0.33 |
| 9 | 3c | nd d | nd d | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valls, A.; Andreu, J.J.; Falomir, E.; Luis, S.V.; Atrián-Blasco, E.; Mitchell, S.G.; Altava, B. Imidazole and Imidazolium Antibacterial Drugs Derived from Amino Acids. Pharmaceuticals 2020, 13, 482. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120482
Valls A, Andreu JJ, Falomir E, Luis SV, Atrián-Blasco E, Mitchell SG, Altava B. Imidazole and Imidazolium Antibacterial Drugs Derived from Amino Acids. Pharmaceuticals. 2020; 13(12):482. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120482
Chicago/Turabian StyleValls, Adriana, Jose J. Andreu, Eva Falomir, Santiago V. Luis, Elena Atrián-Blasco, Scott G. Mitchell, and Belén Altava. 2020. "Imidazole and Imidazolium Antibacterial Drugs Derived from Amino Acids" Pharmaceuticals 13, no. 12: 482. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120482