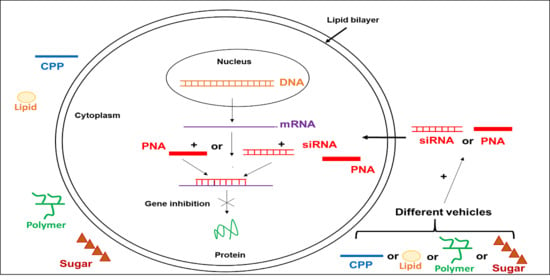
Cell Penetrating Peptides Used in Delivery of Therapeutic Oligonucleotides Targeting Hepatitis B Virus
Abstract
:1. Introduction
2. Antiviral Activity of PNAs Alone on Hepatitis B Virus
3. Anti-HBV Effect of siRNA Alone
4. Benefit of CPPs Used as Vehicles in Oligonucleotides Delivery
4.1. Inhibitory Effect of CPP-PNA Conjugates on Hepadnavirus Replication
4.2. CPP-siRNA Conjugates Decrease Viral Replication
4.3. CPP Alone as Potential Anti-HBV Drugs
5. Effect of These Oligonucleotides on Others Viruses
6. Other Oligonucleotide Delivery Systems
6.1. Sugar-Oligonucleotide Conjugates
6.2. Lipid-Oligonucleotide Conjugates
6.3. Polymer-Oligonucleotide Conjugates
6.4. Aptamer-Oligonucleotide Conjugates
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EASL 2017. Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Hepatitis Report; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Prescott, N.A.; Bram, Y.; Schwartz, R.E.; David, Y. Targeting Hepatitis B Virus Covalently Closed Circular DNA and Hepatitis B Virus X Protein: Recent Advances and New Approaches. ACS Infect. Dis. 2019, 5, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.Y.; Alexander, G.J.; Alberti, A. Viral Hepatitis. Gut Suppl. 1991, S47–S62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybicka, M.; Bielawski, K.P. Recent Advances in Understanding, Diagnosing, and Treating Hepatitis B Virus Infection. Microorganisms 2020, 8, 1416. [Google Scholar] [CrossRef] [PubMed]
- Fourati, S.; Pawlotsky, J.M. Recent advances in understanding and diagnosing hepatitis B virus infection. Research 2016, 5, 2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef]
- Egholm, M.; Buchardt, O.; Christensen, L.; Behrens, C.; Freier, S.M.; Driver, D.A.; Berg, R.H.; Kim, S.K.; Norden, B.; Nielsen, P.E. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature 1993, 365, 566–568. [Google Scholar] [CrossRef]
- Robaczewska, M.; Narayan, R.; Seigneres, B.; Schorr, O.; Thermet, A.; Podhajska, A.J.; Trepo, C.; Zoulim, F.; Nielsen, P.E.; Cova, L. Sequence-specific inhibition of duck hepatitis B virus reverse transcription by peptide nucleic acids (PNA). J. Hepatol. 2005, 42, 180–187. [Google Scholar] [CrossRef]
- Dinca, A.; Chien, W.M.; Chin, M.T. Intracellular Delivery of Proteins with Cell-Penetrating Peptides for Therapeutic Uses in Human Disease. Int. J. Mol. Sci. 2016, 17, 263. [Google Scholar] [CrossRef]
- Ndeboko, B.; Lemamy, G.J.; Nielsen, P.E.; Cova, L. Therapeutic Potential of Cell Penetrating Peptides (CPPs) and Cationic Polymers for Chronic Hepatitis B. Int. J. Mol. Sci. 2015, 12, 28230–28241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndeboko, B.; Ramamurthy, N.; Lemamy, G.J.; Jamard, C.; Nielsen, P.E.; Cova, L. Role of Cell-Penetrating Peptides in Intracellular Delivery of Peptide Nucleic Acids Targeting Hepadnaviral Replication. Mol. Ther. Nucleic Acids 2017, 16, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttolomondo, M.; Casella, C.; Hansen, P.L.; Polo, E.; Herda, L.M.; Dawson, K.A.; Ditzel, H.J.; Mollenhauer, J. Human DMBT1-Derived Cell-Penetrating Peptides for Intracellular siRNA Delivery. Mol. Ther. Nucleic Acids 2017, 8, 264–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus TAT trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the TAT protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [PubMed]
- Joliot, A.; Pernelle, C.; Deagostini-Bazin, H.; Prochiantz, A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 1864–1868. [Google Scholar] [CrossRef] [Green Version]
- Elliott, G.; O’Hare, P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell 1997, 88, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Pooga, M.; Soomets, U.; Hallbrink, M.; Valkna, A.; Saar, K.; Rezaei, K.; Kahl, U.; Hao, J.-X.; Xu, X.-J.; Wiesenfeld-Hallin, Z.; et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat. Biotechnol. 1998, 16, 857–861. [Google Scholar] [CrossRef]
- Wender, P.A.; Mitchell, D.J.; Pattabiraman, K.; Pelkey, E.T.; Steinman, L.; Rothbard, J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13003–13008. [Google Scholar] [CrossRef] [Green Version]
- Heitz, F.; Morris, M.C.; Divita, G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009, 157, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Montrose, K.; Yang, Y.; Krissansen, G.W. X-pep, a novel cell-penetrating peptide motif derived from the hepatitis B virus. Biochem. Biophys. Res. Commun. 2014, 453, 64–68. [Google Scholar] [CrossRef]
- Karolina, D.S.; Jeyaseelan, K. microRNAs as therapeutic agents and targets. In Advanced Delivery and Therapeutic Applications of RNAi; Cheng, K., Mahato, R.I., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2009; pp. 439–482. [Google Scholar]
- Bhattacharjee, S.; Roche, B.; Martienssen, R.A. RNA-induced initiation of transcriptional silencing (RITS) complex structure and function. RNA Biol. 2019, 16, 1133–1146. [Google Scholar] [CrossRef]
- Zhang, X.N.; Xiong, W.; Wang, J.D.; Hu, Y.W.; Xiang, L.; Yuan, Z.H. siRNA-mediated inhibition of HBV replication and expression. World J. Gastroenterol. 2004, 10, 2967–2971. [Google Scholar] [CrossRef]
- Reynolds, N.; Dearnley, M.; Hinton, T.M. Polymers in the Delivery of siRNA for the Treatment of Virus Infections. Top. Curr. Chem. 2017, 375, 38. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors Applied for RNAi-Based Antiviral Therapy. Viruses 2020, 12, 924. [Google Scholar] [CrossRef]
- Duraisamy, G.S.; Bhosale, D.; Lipenská, I.; Huvarova, I.; Růžek, D.; Windisch, M.P.; Miller, A.D. Advanced Therapeutics, Vaccinations, and Precision Medicine in the Treatment and Management of Chronic Hepatitis B Viral Infections; Where Are We and Where Are We Going? Viruses 2020, 12, 998. [Google Scholar] [CrossRef]
- Lehto, T.; Ezzat, K.; Wood, M.J.A.; El Andaloussi, S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 15 Pt A, 172–182. [Google Scholar] [CrossRef]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016, 44, 6518–6548. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.P.; Gutfreund, K.S.; Tyrrell, D.L. Lamivudine resistance in hepatitis B: Mechanisms and clinical implications. Drug Resist. Updat. 2001, 4, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Junker-Niepmann, M.; Bartenschlager, R.; Schaller, H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990, 9, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Nassal, M.; Junker-Niepmann, M.; Schaller, H. Translational inactivation of RNA function: Discrimination against a subset of genomic transcripts during HBV nucleocapsid assembly. Cell 1990, 63, 1357–1363. [Google Scholar] [CrossRef]
- Wang, G.H.; Seeger, C. Novel mechanism for reverse transcription in hepatitis B viruses. J. Virol. 1993, 67, 6507–6512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Chan, C.Y.; He, M.L. RNA interference and antiviral therapy. World J. Gastroenterol. 2007, 13, 5169–5179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.L.; Zhang, X.; Zhang, J.; Yang, Y.; Mu, Y.X.; Liu, M.; Lu, L.; Li, Y.; Zhu, Y.; Wu, J. Inhibition of Hepatitis B virus gene expression by single and dual small interfering RNA treatment. Virus Res. 2005, 112, 100–107. [Google Scholar] [CrossRef]
- Ndeboko, B.; Hantz, O.; Lemamy, G.J.; Cova, L. Developments in Cell-Penetrating Peptides as Antiviral Agents and as Vehicles for Delivery of Peptide Nucleic Acid Targeting Hepadnaviral Replication Pathway. Biomolecules 2018, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Lonn, P.; Dowdy, S.F. Cationic PTD/CPP-mediated macromolecular delivery: Charging into the cell. Expert Opin. Drug Deliv. 2015, 12, 1627–1636. [Google Scholar] [CrossRef]
- Ye, J.; Liu, E.; Gong, J.; Wang, J.; Huang, Y.; He, H.; Yang, V.C. High-Yield Synthesis of Monomeric LMWP(CPP)-siRNA Covalent Conjugate for Effective Cytosolic Delivery of siRNA. Theranostics 2017, 7, 2495–2508. [Google Scholar] [CrossRef]
- Li, G.Q.; Xu, W.Z.; Wang, J.X.; Deng, W.W.; Li, D.; Gu, H.X. Combination of small interfering RNA and lamivudine on inhibition of human B virus replication in HepG2.2.15 cells. World J. Gastroenterol. 2007, 13, 2324–2327. [Google Scholar] [CrossRef]
- Dinis Ano Bom, A.P.; da Costa Neves, P.C.; Bonacossa de Almeida, C.E.; Silva, D.; Missailidis, S. Aptamers as Delivery Agents of siRNA and Chimeric Formulations for the Treatment of Cancer. Pharmaceutics 2019, 11, 684. [Google Scholar] [CrossRef] [Green Version]
- Osborn, M.F.; Coles, A.H.; Biscans, A.; Haraszti, R.A.; Roux, L.; Davis, S.; Ly, S.; Echeverria, D.; Hassler, M.R.; Godinho, B.; et al. Hydrophobicity drives the systemic distribution of lipid-conjugated siRNAs via lipid transport pathways. Nucleic Acids Res. 2019, 47, 1070–1081. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, P.V.; Gunathunge, B.G.; Wimalasiri, P.N.; Karunaratne, D.N.; Ranatunga, R.J. Trends in the Binding of Cell Penetrating Peptides to siRNA: A Molecular Docking Study. J. Biophys. 2017, 2017, 1059216. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Sato, Y.; Munakata, T.; Kakuni, M.; Tateno, C.; Sanada, T.; Hirata, Y.; Murakami, S.; Tanaka, Y.; Chayama, K.; et al. Novel pH-sensitive multifunctional envelope-type nanodevice for siRNAbased treatments for chronic HBV infection. J. Hepatol. 2016, 64, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y.; Matsui, H.; Yamamoto, N.; Sato, R.; Munakata, T.; Kohara, M.; Harashima, H. Highly specific delivery of siRNA to hepatocytes circumvents endothelial cellmediated lipid nanoparticle-associated toxicity leading to the safe and efficacious decrease in the hepatitis B virus. J. Control. Release 2017, 266, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Zahid, M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics 2020, 12, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manda, V.; Josyula, V.R.; Hariharapura, R.C. siRNA intervention inhibiting viral replication and delivery strategies for treating herpes simplex viral infection. Virusdisease 2019, 30, 180–185. [Google Scholar] [CrossRef]
- Egal, M.; Conrad, M.; MacDonald, D.L.; Maloy, W.L.; Motley, M.; Genco, C.A. Antiviral effects of synthetic membrane-active peptides on herpes simplex virus, type 1. Int. J. Antimicrob. Agents 1999, 13, 57–60. [Google Scholar] [CrossRef]
- Albiol Matanic, V.C.; Castilla, V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents 2004, 23, 382–389. [Google Scholar] [CrossRef]
- Agarwal, G.; Gabrani, R. Antiviral Peptides: Identification and Validation. Int. J. Pept. Res. Ther. 2020, 18, 1–20. [Google Scholar] [CrossRef]
- Abdul, F.; Ndeboko, B.; Buronfosse, T.; Zoulim, F.; Kann, M.; Nielsen, P.E.; Cova, L. Potent inhibition of late stages of hepadnavirus replication by a modified cell penetrating peptide. PLoS ONE 2012, 7, e48721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacque, J.M.; Triques, K.; Stevenson, M. Modulation of HIV-1 replication by RNA interference. Nature 2002, 418, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qian, Q.; Shu, T.; Xu, J.; Kong, J.; Mu, J.; Qiu, Y.; Zhou, X. Hepatitis C Virus NS2 Protein Suppresses RNA Interference in Cells. Virol Sin. 2020, 35, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljowaie, R.M.; Almajhdi, F.N.; Ali, H.H.; El-Wetidy, M.S.; Shier, M.K. Inhibition of hepatitis C virus genotype 4 replication using siRNA targeted to the viral core region and the CD81 cellular receptor. Cell Stress Chaperones 2020, 25, 345–355. [Google Scholar] [CrossRef]
- Nulf, C.J.; Corey, D. Intracellular inhibition of hepatitis C virus (HCV) internal ribosomal entry site (IRES)-dependent translation by peptide nucleic acids (PNAs) and locked nucleic acids (LNAs). Nucleic Acids Res. 2004, 32, 3792–3798. [Google Scholar] [CrossRef]
- He, M.L.; Zheng, B.; Peng, Y.; Peiris, J.S.; Poon, L.L.; Yuen, K.Y.; Lin, M.C.; Kung, H.F.; Guan, Y. Inhibition of SARS-associated coronavirus infection and replication by RNA interference. JAMA 2003, 290, 2665–2666. [Google Scholar] [CrossRef] [Green Version]
- Grijalvo, S.; Alagia, A.; Jorge, A.F.; Eritja, R. Covalent Strategies for Targeting Messenger and Non-Coding RNAs: An Updated Review on siRNA, miRNA and antimiR Conjugates. Genes 2018, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef]
- Roberts, T.C.; Langer, R.; Wood, M. Advances in oligonucleotide drug delivery. Nature Rev. Drug Discov. 2020, 19, 673–694. [Google Scholar] [CrossRef]


| Drugs | Gene Delivery System | Target | Cells/Animal Models | Antiviral Effect | Toxicity |
|---|---|---|---|---|---|
| PNA alone | - | Duck HBV | PDH cell cultures | + | − [9] |
| CPP-PNA | CPP | Duck HBV | PDH cell cultures Ducklings | + | − [9,12] |
| siRNA alone | - | HBV | Huh-7 cells Transgenic mouse HepG2.2.15 | + | − [25,26,27] |
| CPP-siRNA | CPP | HBV | Transgenic mouse HepG2.2.15 | + | − [13,28,48] |
| CPP | - | Duck HBV | PDH cell cultures Ducklings | + | − [12] |
| Modified CPP | - | Duck HBV HBV | PDH cell cultures HepG2.2.15 cells | + | − [53] |
Publisher‘s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndeboko, B.; Omouessi, S.T.; Ongali, B.; Mouinga-Ondémé, A. Cell Penetrating Peptides Used in Delivery of Therapeutic Oligonucleotides Targeting Hepatitis B Virus. Pharmaceuticals 2020, 13, 483. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120483
Ndeboko B, Omouessi ST, Ongali B, Mouinga-Ondémé A. Cell Penetrating Peptides Used in Delivery of Therapeutic Oligonucleotides Targeting Hepatitis B Virus. Pharmaceuticals. 2020; 13(12):483. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120483
Chicago/Turabian StyleNdeboko, Bénédicte, Serge Thierry Omouessi, Brice Ongali, and Augustin Mouinga-Ondémé. 2020. "Cell Penetrating Peptides Used in Delivery of Therapeutic Oligonucleotides Targeting Hepatitis B Virus" Pharmaceuticals 13, no. 12: 483. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120483