Folate and Borneol Modified Bifunctional Nanoparticles for Enhanced Oral Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of FA-PEI Copolymer
2.3. Synthesis and Characterization of BO-PEI Copolymer
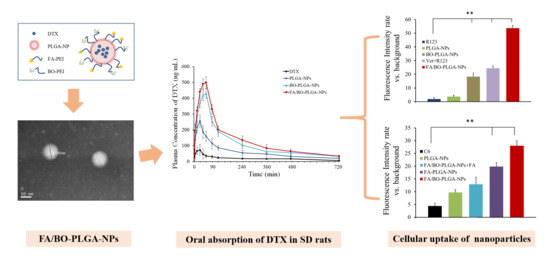
2.4. Preparation and Characterization of FA/BO-PLGA-NPs
2.5. Stability and In Vitro Release of FA/BO-PLGA-NPs
2.6. HPLC Analytical Method
2.7. Intestinal Absorption
2.8. Cell Culture
2.9. Cell Cytotoxicity Assay
2.10. Cellular Uptake Mechanism Studies of Bifunctional Nanoparticles In Vitro
2.11. Pharmacokinetics Study
2.12. Intestinal Irritation of FA/BO-PLGA-NPs
2.13. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of FA-PEI Copolymer
3.2. Synthesis and Characterization of BO-PEI Copolymer
3.3. Characterization of FA/BO-PLGA-NPs
3.4. Stability and In Vitro Release of FA/BO-PLGA-NPs
3.5. Intestinal Absorption
3.6. Cell Cytotoxicity Assay
3.7. Cellular Uptake Mechanism Studies of Bifunctional Nanoparticles
3.8. Pharmacokinetics Study
3.9. Intestinal Irritation of FA/BO-PLGA-NPs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, Y.; Chu, M.; Tan, S.; Zhao, S.; Liu, H.; Otieno, B.O.; Yang, X.; Xu, C.; Zhang, Z. Chitosan-g-tpgs nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol. Pharm. 2013, 11, 59–70. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Shen, Q.; Li, J. Effects of borneol on the intestinal transport and absorption of two p-glycoprotein substrates in rats. Arch. Pharm. Res. 2011, 34, 1161. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Nurunnabi, M.; Cho, K.J.; Byun, Y.; Bae, Y.H.; Lee, Y.K. Oral absorption mechanism and anti-angiogenesis effect of taurocholic acid-linked heparin-docetaxel conjugates. J. Control. Release 2014, 177, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Montero, A.; Fossella, F.; Hortobagyi, G.; Valero, V. Docetaxel for treatment of solid tumours: A systematic review of clinical data. Lancet Oncol. 2005, 6, 229–239. [Google Scholar] [CrossRef]
- Feng, S.S.; Shu, C. Chemotherapeutic engineering: Application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003, 58, 4087–4114. [Google Scholar] [CrossRef]
- Baker, J.; Ajani, J.; Scotte, F.; Winther, D.; Martin, M.; Aapro, M.S.; von Minckwitz, G. Docetaxel-related side effects and their management. Eur. J. Oncol. Nurs. 2009, 13, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.; Ernsting, M.J.; Murakami, M.; Undzys, E.; Li, S.D. Docetaxel-carboxymethylcellulose nanoparticles display enhanced anti-tumor activity in murine models of castration-resistant prostate cancer. Int. J. Pharm. 2014, 471, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, A.; Attar, H.; Seyf Kordi, A.A.; Iman, M.; Jaafari, M.R. New oral formulation and in vitro evaluation of docetaxel-loaded nanomicelles. Molecules 2016, 21, 1265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Astruc, D. Docetaxel nanotechnology in anticancer therapy. ChemMedChem 2012, 7, 952–972. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Cao, S.; Hu, F.; Feng, J. Enhanced oral bioavailability of docetaxel by lecithin nanoparticles: Preparation, in vitro, and in vivo evaluation. Int. J. Nanomed. 2012, 7, 3537–3545. [Google Scholar] [CrossRef] [PubMed]
- Saremi, S.; Atyabi, F.; Akhlaghi, S.P.; Ostad, S.N.; Dinarvand, R. Thiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: Preparation, in vitro and ex vivo evaluation. Int. J. Nanomed. 2011, 6, 119–128. [Google Scholar]
- Kumar, G.; Shafiq, N.; Malhotra, S. Drug-loaded PLGA nanoparticles for oral administration: Fundamental issues and challenges ahead. Crit. Rev. Ther. Drug 2012, 29, 149–182. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, M.; Zheng, A.; Cao, D.; Bi, Y.; Sun, J. Preparation and characterization of insulin-loaded bioadhesive PLGA nanoparticles for oral administration. Eur. J. Pharm. Sci. 2012, 45, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine) and its role in gene delivery. J. Control. Release 1999, 60, 149–160. [Google Scholar] [CrossRef]
- Kim, T.H.; Akaike, T.; Chong, S.C. Synergistic effect of poly(ethylenimine) on the transfection efficiency of galactosylated chitosan/DNA complexes. J. Control. Release 2005, 105, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Fang, G.; Gao, Y.; Liu, Y.; Liu, J.; Zou, M.; Wang, L.; Cheng, G. Lipid-albumin nanoassemblies co-loaded with borneol and paclitaxel for intracellular drug delivery to c6 glioma cells with p-gp inhibition and its tumor targeting. Asian J. Pharm. Sci. 2015, 10, 363–371. [Google Scholar] [CrossRef]
- Ru, G.; Han, L.; Qing, J.; Sheng, J.; Li, R.; Qiu, M.; Wang, J. Effects of borneol on the pharmacokinetics of 9-nitrocamptothecin encapsulated in PLGA nanoparticles with different size via oral administration. Drug Deliv. 2016, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gong, X.; Lu, Y.; Du, S.; Yang, Z.; Bai, J.; Li, P.; Wu, H. Enhancing effect of borneol and muscone on geniposide transport across the human nasal epithelial cell monolayer. PLoS ONE 2014, 9, e101414. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Yokoyama, T.; Masaoka, Y.; Sakuma, S.; Yamashita, S. Estimation of p-glycoprotein-mediated efflux in the oral absorption of p-gp substrate drugs from simultaneous analysis of drug dissolution and permeation. Eur. J. Pharm. Sci. 2011, 44, 544. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Savin, M.A.; Holmes, F.A.; O’Shaughnessy, J.A.; Blum, J.L.; Vukelja, S.; Mcintyre, K.J.; Pippen, J.E.; Bordelon, J.H.; Kirby, R. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J. Clin. Oncol. 2006, 18, 5381–5387. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.D.A.; Ratnam, M. The folate receptor: What does it promise in tissue-targeted therapeutics? Cancer Metast. Rev. 2007, 26, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Wang, X.; Zhu, W.; Zhang, C.; Song, A.; Sun, C.; Jiang, T.; Wang, S. Folate-polyethyleneimine functionalized mesoporous carbon nanoparticles for enhancing oral bioavailability of paclitaxel. Int. J. Pharm. 2015, 484, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Roger, E.; Kalscheuer, S.; Kirtane, A.; Guru, B.R.; Grill, A.E.; Whittumhudson, J.; Panyam, J. Folic acid functionalized nanoparticles for enhanced oral drug delivery. Mol. Pharm. 2012, 9, 2103–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visentin, M.; Diopbove, N.; Zhao, R.; Goldman, I.D. The intestinal absorption of folates. Annu. Rev. Physiol. 2014, 76, 251–274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.B.; Matherly, L.H.; Goldman, I.D. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 2009, 11, e4. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, B.L.; Gregor, J.C.; Chande, N.; Knauer, M.J.; Tirona, R.G.; Kim, R.B. The human proton-coupled folate transporter (hpcft): Modulation of intestinal expression and function by drugs. Am. J. Physiol. 2010, 298, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Goldman, I.D. The molecular identity and characterization of a proton-coupled folate transporter-pcft; biological ramifications and impact on the activity of pemetrexed. Cancer Metast. Rev. 2007, 26, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.; Yun, W.; Xu, S.; Xiao, Z.; Min, L.; Liu, Q.; Shen, Y.; Cui, D.; Guo, S. Preparation and evaluation of pH-responsive charge-convertible ternary complex fa-pei-cca/pei/DNA with low cytotoxicity and efficient gene delivery. Colloids Surf. B 2017, 152, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Guo, M.M.; Han, S.P.; Sun, Y.; Fei, W.D.; Xu, X.I.; Li, F.Z. preparation and in vitro evaluation of borneol and folic acid co-modified doxorubicin loaded pamam drug delivery system. Acta Pharm. Sin. 2015, 50, 899. [Google Scholar]
- Xinyi, J.; Hongliang, X.; Qiuyue, R.; Jijin, G.; Lingjun, Z.; Fengyi, D.; Chunlai, F.; Yike, X.; Xianyi, S.; Xiaoling, F. Nanoparticles of 2-deoxy-d-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials 2014, 35, 518–529. [Google Scholar]
- Chen, Y.S.; Alany, R.G.; Young, S.A.; Green, C.R.; Rupenthal, I.D. In vitro release characteristics and cellular uptake of poly(d,l-lactic-co-glycolic acid) nanoparticles for topical delivery of antisense oligodeoxynucleotides. Drug Deliv. 2011, 18, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Sun, J.; Cao, W.; Yang, J.H.; Lian, H.; Li, X.; Sun, Y.H.; Wang, Y.J.; Wang, S.L.; He, Z.G. Dual targeting folate-conjugated hyaluronic acid polymeric micelles for paclitaxel delivery. Int. J. Pharm. 2011, 421, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, T.; Imai, K.; Nezu, J.I.; Tsuji, A.; Tamai, I. Functional characterization of pH-sensitive organic anion transporting polypeptide oatp-b in human. J. Pharmacol. Exp. Ther. 2004, 308, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, J.J.; Zair, Z.M.; Hiller, C.; Hausler, S.; Stieger, B.; Kullak-Ublick, G.A. Vitamin d-3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter (vol 76, pg 1062, 2009). Mol. Pharmacol. 2010, 77, 885. [Google Scholar]
- Ahram, M.; Flaig, M.J.; Gillespie, J.W.; Duray, P.H.; Linehan, W.M.; Ornstein, D.K.; Niu, S.L.; Zhao, Y.M.; Petricoin, E.F.; Emmert-Buck, M.R. Evaluation of ethanol-fixed, paraffin-embedded tissues for proteomic applications. Proteomics 2003, 3, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. Aaps Pharmscitech 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Waterschoot, R.A.B.; Lagas, J.S.; Wagenaar, E.; Rosing, H.; Beijnen, J.H.; Schinkel, A.H. Individual and combined roles of cyp3a, p-glycoprotein (mdr1/abcb1) and mrp2 (abcc2) in the pharmacokinetics of docetaxel. Int. J. Cancer 2010, 127, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tomás, R. Multidrug resistance: Retrospect and prospects in anti-cancer drug treatment. Curr. Med. Chem. 2006, 13, 1859–1876. [Google Scholar] [CrossRef]
- Cai, Z.; Hou, S.; Li, Y.; Zhao, B.; Yang, Z.; Xu, S.; Pu, J. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration, journal of drug targeting, informa healthcare. J. Drug Target 2008, 16, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Z.; Lu, Y.; Du, S.Y.; Shang, K.X.; Cai, C.B. Influence of borneol and muscone on geniposide transport through mdck and mdck-mdr1 cells as blood-brain barrier in vitro model. Int. J. Pharm. 2013, 456, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Li, R.; Wang, P.; Xiao, Y.; Xu, L.J.; He, Y.X.; Zhao, G.; Peng, L.X. The study of absorption kinetics of berberine based on portal vein in rat, and the influence of verapamil and borneol to its absorption ability by uhplc method. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Cartiera, M.S.; Johnson, K.M.; Rajendran, V.; Caplan, M.J.; Saltzman, W.M. The uptake and intracellular fate of PlGA nanoparticles in epithelial cells. Biomaterials 2009, 30, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Jansen, M.; Sakaris, A.; Min, S.H.; Chattopadhyay, S.; Tsai, E.; Sandoval, C.; Zhao, R.; Akabas, M.H.; Goldman, I.D. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 2006, 127, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Keating, E.; Lemos, C.; Azevedo, I.; Martel, F. Comparison of folic acid uptake characteristics by human placental choriocarcinoma cells at acidic and physiological pH. Can. J. Physiol. Pharmacol. 2006, 84, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Jaitley, V.; Florence, A.T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv. Drug Deliv. Rev. 2001, 50, 107–142. [Google Scholar] [CrossRef]
- Chen, J.; He, H.; Li, S.; Shen, Q. An hplc method for the pharmacokinetic study of vincristine sulfate-loaded plga-peg nanoparticle formulations after injection to rats. J. Chromatogr. B 2011, 879, 1967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Shen, Q.; Ma, Y. An hplc method for the pharmacokinetic study of daidzein-loaded nanoparticle formulations after injection to rats. J. Chromatogr. B 2011, 879, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, K.; Zhou, Y.; Ding, L.; Ullah, A.; Hu, Q.; Sun, M.; Oupický, D. Oral nanostructured lipid carriers loaded with near-infrared dye for image-guided photothermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 25087–25095. [Google Scholar] [CrossRef] [PubMed]











| Formulation | Papp (* 10−6 cm/s) (Mean ± SD) | Absorption Promoting Ratio (R) |
|---|---|---|
| DTX | 1.4 ± 1.1 | 1 |
| PLGA-NPs | 3.7 ± 0.6 * | 2.7 * |
| FA-PLGA-NPs | 7.6 ± 1.9 ** | 5.5 ** |
| BO-PLGA-NPs | 6.9 ± 0.5 ** | 4.9 ** |
| FA/BO-PLGA-NPs | 7.8 ± 3.9 ** | 6.0 ** |
| Groups | IC50 (μg/mL) ± SD |
|---|---|
| DTX | 178.20 ± 0.50 |
| PLGA-NPs | 28.70 ± 0.21 ** |
| BO-PLGA-NPs | 9.66 ± 0.09 ** |
| FA-PLGA-NPs | 8.81 ± 0.10 ** |
| FA/BO-PLGA-NPs | 6.99 ± 0.08 ** |
| DTX | PLGA-NPs | BO-PLGA-NPs | FA/BO-PLGA-NPs | |
|---|---|---|---|---|
| Cmax (mg/L) | 0.07 ± 0.87 | 0.26 ± 0.04 ** | 0.43 ± 1.01 ** | 0.50 ± 0.53 ** |
| Tmax (h) | 0.44 ± 0.00 | 0.57 ± 0.03 | 0.92 ± 0.05 ** | 1.06 ± 0.09 ** |
| AUC0–12h (mg/L*h) | 0.23 ± 0.12 | 0.64 ± 0.14 ** | 1.07 ± 0.02 ** | 1.57 ± 0.60 ** |
| Frel (%) | / | 278.3 | 465.2 | 682.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Yin, Y.; Zhang, J.; Zuo, T.; Liang, X.; Li, J.; Shen, Q. Folate and Borneol Modified Bifunctional Nanoparticles for Enhanced Oral Absorption. Pharmaceutics 2018, 10, 146. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030146
Yang Y, Yin Y, Zhang J, Zuo T, Liang X, Li J, Shen Q. Folate and Borneol Modified Bifunctional Nanoparticles for Enhanced Oral Absorption. Pharmaceutics. 2018; 10(3):146. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030146
Chicago/Turabian StyleYang, Yifan, Yunzhi Yin, Jun Zhang, Tiantian Zuo, Xiao Liang, Jing Li, and Qi Shen. 2018. "Folate and Borneol Modified Bifunctional Nanoparticles for Enhanced Oral Absorption" Pharmaceutics 10, no. 3: 146. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10030146