Probing the Action of Permeation Enhancers Sodium Cholate and N-dodecyl-β-D-maltoside in a Porcine Jejunal Mucosal Explant System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Organ Culture of Porcine Jejunal Mucosa
2.4. Fluorescence Microscopy
2.5. Transmission Electron Microscopy
2.6. Preparation of Microvillus Membrane Vesicles and Treatment with NaC and DDM
2.7. Detergent Resistant Membrane (DRM) Analysis of Microvillus Membranes by Sucrose Gradient Ultracentrifugation
2.8. SDS/PAGE and Immunoblotting
3. Results
3.1. Microvillus Membrane-Solubilizing Effects of NaC and DDM
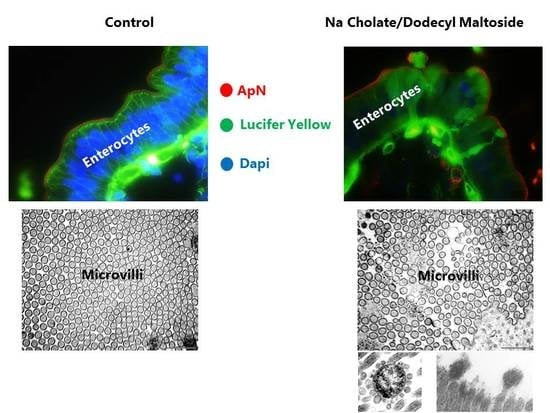
3.2. Effects of NaC and DDM on Mucosal Morphology in Cultured Mucosal Explants
3.3. Effects of NaC and DDM on Permeability of Lucifer Yellow
3.4. Effects of NaC and DDM on Permeability of Texas Red Dextran and FM 1-43 FX
3.5. Effects of NaC and DDM on the Ultrastructure of the Enterocyte Brush Border
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crawley, S.W.; Mooseker, M.S.; Tyska, M.J. Shaping the intestinal brush border. J. Cell Biol. 2014, 207, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delacour, D.; Salomon, J.; Robine, S.; Louvard, D. Plasticity of the brush border—The yin and yang of intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, K.; Carlsen, J. Microvillus membrane vesicles from pig small intestine. Purity and lipid composition. Biochim. Biophys. Acta 1981, 647, 188–195. [Google Scholar] [CrossRef]
- Danielsen, E.M.; Hansen, G.H. Lipid rafts in epithelial brush borders: Atypical membrane microdomains with specialized functions. Biochim. Biophys. Acta 2003, 1617, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Mrsny, R.J.; Brayden, D.J. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 277–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenberg, D.; Robson, R.J.; Dennis, E.A. Solubilization of phospholipids by detergents. Structural and kinetic aspects. Biochim. Biophys. Acta 1983, 737, 285–304. [Google Scholar] [CrossRef]
- McCartney, F.; Gleeson, J.P.; Brayden, D.J. Safety concerns over the use of intestinal permeation enhancers: A mini-review. Tissue Barriers 2016, 4, e1176822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundquist, P.; Artursson, P. Oral absorption of peptides and nanoparticles across the human intestine: Opportunities, limitations and studies in human tissues. Adv. Drug Deliv. Rev. 2016, 106, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Navarro, M.; Garcia, J.; Giralt, E.; Teixido, M. Using peptides to increase transport across the intestinal barrier. Adv. Drug Deliv. Rev. 2016, 106, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Shiau, Y.F. Mechanisms of intestinal fat absorption. Am. J. Physiol. 1981, 240, G1–G9. [Google Scholar] [CrossRef] [PubMed]
- Aungst, B.J. Absorption enhancers: Applications and advances. AAPS J. 2012, 14, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Fasinu, P.; Pillay, V.; Ndesendo, V.M.; du Toit, L.C.; Choonara, Y.E. Diverse approaches for the enhancement of oral drug bioavailability. Biopharm. Drug Dispos. 2011, 32, 185–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yewale, C.; Patil, S.; Kolate, A.; Kore, G.; Misra, A. Oral Absorption Promoters: Opportunities, Issues, and Challenges. Crit. Rev. Ther. Drug Carr. Syst. 2015, 32, 363–387. [Google Scholar] [CrossRef]
- Petersen, S.B.; Nolan, G.; Maher, S.; Rahbek, U.L.; Guldbrandt, M.; Brayden, D.J. Evaluation of alkylmaltosides as intestinal permeation enhancers: Comparison between rat intestinal mucosal sheets and Caco-2 monolayers. Eur. J. Pharm. Sci. 2012, 47, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, U.S.; Hansen, G.H.; Danielsen, E.M. Organ Culture as a Model System for Studies on Enterotoxin Interactions with the Intestinal Epithelium. Methods Mol. Biol. 2016, 1396, 159–166. [Google Scholar] [PubMed]
- Danielsen, E.T.; Danielsen, E.M. Glycol chitosan: A stabilizer of lipid rafts in the intestinal brush border. Biochim. Biophys. Acta 2017, 1859, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, E.M.; Hansen, G.H. Intestinal surfactant permeation enhancers and their interaction with enterocyte cell membranes in a mucosal explant system. Tissue Barriers 2017, 5, e1361900. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, E.M.; Hansen, G.H. Impact of cell-penetrating peptides (CPPs) melittin and Hiv-1 Tat on the enterocyte brush border using a mucosal explant system. Biochim. Biophys. Acta 2018, 1860, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.H.; Sjostrom, H.; Noren, O.; Dabelsteen, E. Immunomicroscopic localization of aminopeptidase N in the pig enterocyte. Implications for the route of intracellular transport. Eur. J. Cell Biol. 1987, 43, 253–259. [Google Scholar] [PubMed]
- Danielsen, E.M.; Sjostrom, H.; Noren, O.; Bro, B.; Dabelsteen, E. Biosynthesis of intestinal microvillar proteins. Characterization of intestinal explants in organ culture and evidence for the existence of pro-forms of the microvillar enzymes. Biochem. J. 1982, 202, 647–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, A.G.; Kenny, A.J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem. J. 1974, 142, 575–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, D.A.; Rose, J.K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992, 68, 533–544. [Google Scholar] [CrossRef]
- Danielsen, E.M. Involvement of detergent-insoluble complexes in the intracellular transport of intestinal brush border enzymes. Biochemistry 1995, 34, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology 2006, 21, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M. Lucifer yellow—An angel rather than the devil. J. Cell. Mol. Med. 2012, 16, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, E.M.; Hansen, G.H.; Rasmussen, K.; Niels-Christiansen, L.L. Permeabilization of enterocytes induced by absorption of dietary fat. Mol. Membr. Biol. 2013, 30, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.H.; Rasmussen, K.; Niels-Christiansen, L.L.; Danielsen, E.M. Endocytic trafficking from the small intestinal brush border probed with FM dye. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G708–G715. [Google Scholar] [CrossRef] [PubMed]
- Woodcroft, B.J.; Hammond, L.; Stow, J.L.; Hamilton, N.A. Automated organelle-based colocalization in whole-cell imaging. Cytom. A 2009, 75, 941–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolte, S.; Talbot, C.; Boutte, Y.; Catrice, O.; Read, N.D.; Satiat-Jeunemaitre, B. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc. 2004, 214, 159–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.R.; Campana, M.; Stolnik, S.; Lam, J.K.W.; Casettari, L.; Duranti, A. Synthesis, Structure(-)Activity Relationships and In Vitro Toxicity Profile of Lactose-Based Fatty Acid Monoesters as Possible Drug Permeability Enhancers. Pharmaceutics 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Fagioli, L.; Campana, R.; Cole, H.; Duranti, A.; Baffone, W.; Vllasaliu, D.; Casettari, L. Unsaturated fatty acids lactose esters: Cytotoxicity, permeability enhancement and antimicrobial activity. Eur. J. Pharm. Biopharm. 2016, 107, 88–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blikslager, A.T.; Moeser, A.J.; Gookin, J.L.; Jones, S.L.; Odle, J. Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 2007, 87, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed]
- Mrsny, R.J. Strategies for targeting protein therapeutics to selected tissues and cells. Expert Opin. Biol. Ther. 2004, 4, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Tyska, M.J.; Mackey, A.T.; Huang, J.D.; Copeland, N.G.; Jenkins, N.A.; Mooseker, M.S. Myosin-1a is critical for normal brush border structure and composition. Mol. Biol. Cell 2005, 16, 2443–2457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helander, H.F.; Fandriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Mooseker, M.S.; Graves, T.A.; Wharton, K.A.; Falco, N.; Howe, C.L. Regulation of microvillus structure: Calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J. Cell Biol. 1980, 87, 809–822. [Google Scholar] [CrossRef] [PubMed]
- McConnell, R.E.; Higginbotham, J.N.; Shifrin, D.A., Jr.; Tabb, D.L.; Coffey, R.J.; Tyska, M.J. The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 2009, 185, 1285–1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shifrin, D.A., Jr.; Tyska, M.J. Ready…aim…fire into the lumen: A new role for enterocyte microvilli in gut host defense. Gut Microbes 2012, 3, 460–462. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danielsen, E.M.; Hansen, G.H. Probing the Action of Permeation Enhancers Sodium Cholate and N-dodecyl-β-D-maltoside in a Porcine Jejunal Mucosal Explant System. Pharmaceutics 2018, 10, 172. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10040172
Danielsen EM, Hansen GH. Probing the Action of Permeation Enhancers Sodium Cholate and N-dodecyl-β-D-maltoside in a Porcine Jejunal Mucosal Explant System. Pharmaceutics. 2018; 10(4):172. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10040172
Chicago/Turabian StyleDanielsen, E. Michael, and Gert H. Hansen. 2018. "Probing the Action of Permeation Enhancers Sodium Cholate and N-dodecyl-β-D-maltoside in a Porcine Jejunal Mucosal Explant System" Pharmaceutics 10, no. 4: 172. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics10040172