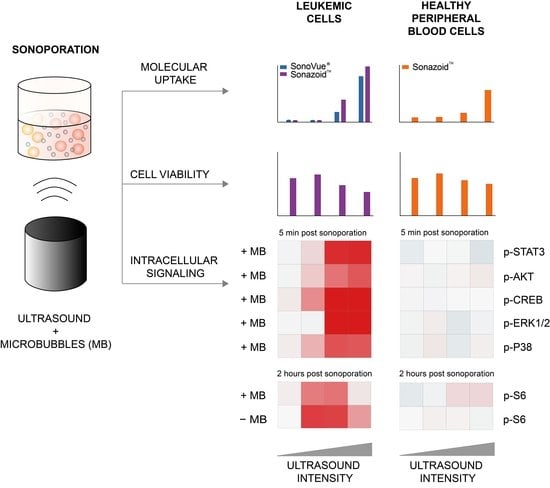
Intracellular Signaling in Key Pathways Is Induced by Treatment with Ultrasound and Microbubbles in a Leukemia Cell Line, but Not in Healthy Peripheral Blood Mononuclear Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microbubbles
2.3. Cell Culture
2.4. In Vitro Treatment with Ultrasound and Microbubbles
2.5. Uptake of Cell Impermeable Dye
2.6. Viability Analysis
2.7. Sample Preparation for Phosphospecific Flow Cytometry
2.8. Barcoding and Antibody Staining
2.9. Western Blots
2.10. Data Analysis/Statistical Analysis
3. Results
3.1. Efficiency of Sonoporation Was Increased by Addition of Microbubbles and High Ultrasound Intensity
3.1.1. Percentage of Cells Taking Up Calcein (Permeabilization Efficiency)
3.1.2. Quantified Uptake of Calcein
3.2. Decreased Viability in Cancerous MOLM-13 Cells after Sonoporation
3.3. Sonoporation Induced Changes in Intracellular Signaling-Profiles
3.3.1. Immediate Effects of Sonoporation
3.3.2. Downstream Effects of Sonoporation—Ribosomal Protein S6
3.3.3. Downstream Effects of Sonoporation—Eukaryotic Initiation Factor 2α
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Escoffre, J.M.; Bouakaz, A. Therapeutic Ultrasound; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Qin, J.; Wang, T.Y.; Willmann, J.K. Sonoporation: Applications for Cancer Therapy. Adv. Exp. Med. Biol. 2016, 880, 263–291. [Google Scholar] [CrossRef] [PubMed]
- Kotopoulis, S.; Delalande, A.; Popa, M.; Mamaeva, V.; Dimcevski, G.; Gilja, O.H.; Postema, M.; Gjertsen, B.T.; McCormack, E. Sonoporation-enhanced chemotherapy significantly reduces primary tumour burden in an orthotopic pancreatic cancer xenograft. Mol. Imaging Biol. 2014, 16, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kotopoulis, S.; Stigen, E.; Popa, M.; Safont, M.M.; Healey, A.; Kvåle, S.; Sontum, P.; Gjertsen, B.T.; Gilja, O.H.; Mc Cormack, E. Sonoporation with Acoustic Cluster Therapy (ACT®) induces transient tumour volume reduction in a subcutaneous xenograft model of pancreatic ductal adenocarcinoma. J. Control. Release 2017, 245, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.M.; Novell, A.; Serrière, S.; LeComte, T.; Bouakaz, A. Irinotecan Delivery by Microbubble-Assisted Ultrasound: In Vitro Validation and a Pilot Preclinical Study. Mol. Pharm. 2013, 10, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Yamaguchi, K.; Feril, L.B.; Endo, H.; Ogawa, K.; Tachibana, K.; Nakayama, J. Synergistic inhibition of malignant melanoma proliferation by melphalan combined with ultrasound and microbubbles. Ultrason. Sonochem. 2011, 18, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; Mc Cormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016, 243, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targeted Delivery of Chemotherapy with Ultrasound and Microbublles. Available online: https://ClinicalTrials.gov/show/NCT03458975 (accessed on 29 March 2018).
- Ultrasound-enhanced Delivery of Chemotherapy to Patients with Liver Metastasis from Breast- and Colorectal Cancer. Available online: https://ClinicalTrials.gov/show/NCT03477019 (accessed on 14 November 2018).
- KVUS at Neoadjuvant CTx of Breast Cancer. Available online: https://ClinicalTrials.gov/show/NCT03385200 (accessed on 28 December 2017).
- Postema, M.; Spiros, K.; Anthony, D.; Odd, H.G. Sonoporation: Why Microbubbles Create Pores. Ultraschall Med. 2012, 11, 97–98. [Google Scholar]
- Chen, X.; Wan, J.M.; Yu, A.C. Sonoporation as a Cellular Stress: Induction of Morphological Repression and Developmental Delays. Ultrasound Med. Biol. 2013, 39, 1075–1086. [Google Scholar] [CrossRef]
- Whitney, N.P.; Lamb, A.C.; Louw, T.M.; Subramanian, A. Integrin-Mediated Mechanotransduction Pathway of Low-Intensity Continuous Ultrasound in Human Chondrocytes. Ultrasound Med. Biol. 2012, 38, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Nagata, K.; Kuroda, S.; Horiuchi, S.; Nakamura, T.; Karima, M.; Inubushi, T.; Tanaka, E. Low-Intensity Pulsed Ultrasound Activates Integrin-Mediated Mechanotransduction Pathway in Synovial Cells. Ann. Biomed. Eng. 2014, 42, 2156–2163. [Google Scholar] [CrossRef]
- Takeuchi, R.; Ryo, A.; Komitsu, N.; Mikuni-Takagaki, Y.; Fukui, A.; Takagi, Y.; Shiraishi, T.; Morishita, S.; Yamazaki, Y.; Kumagai, K.; et al. Low-intensity pulsed ultrasound activates the phosphatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: A basic science study. Arthritis Res. Ther. 2008, 10, R77. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Tang, C.H.; Yang, R.S.; Huang, T.H.; Lu, D.Y.; Chuang, W.J.; Fu, W.M. Ultrasound Stimulates Cyclooxygenase-2 Expression and Increases Bone Formation through Integrin, Focal Adhesion Kinase, Phosphatidylinositol 3-Kinase, and Akt Pathway in Osteoblasts. Mol. Pharmacol. 2006, 69, 2047–2057. [Google Scholar] [CrossRef] [Green Version]
- Kotopoulis, S.; Yddal, T.; Cochran, S.; Gilja, O.H.; Postema, M. Open-source, high-throughput ultrasound treatment chamber. Biomed. Tech. Eng. 2015, 60, 77–87. [Google Scholar] [CrossRef]
- Ter Haar, G. Safety and bio-effects of ultrasound contrast agents. Med. Biol. Eng. Comput. 2009, 47, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Zeghimi, A.; Escoffre, J.M.; Bouakaz, A. Role of endocytosis in sonoporation-mediated membrane permeabilization and uptake of small molecules: A electron microscopy study. Phys. Biol. 2015, 12, 066007. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Krutzik, P.O.; Nolan, G.P. Intracellular phospho-protein staining techniques for flow cytometry: Monitoring single cell signaling events. Cytometry 2003, 55, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sulen, A.; Gullaksen, S.E.; Bader, L.; McClymont, D.W.; Skavland, J.; Gavasso, S.; Gjertsen, B.T. Signaling effects of sodium hydrosulfide in healthy donor peripheral blood mononuclear cells. Pharmacol. Res. 2016, 113, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Krutzik, P.; Nolan, G.P. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat. Methods 2006, 3, 361–368. [Google Scholar] [CrossRef]
- Tyagi, R.; Shahani, N.; Gorgen, L.; Ferretti, M.; Pryor, W.; Chen, P.Y.; Swarnkar, S.; Worley, P.F.; Karbstein, K.; Snyder, S.H.; et al. Rheb Inhibits Protein Synthesis by Activating the PERK-eIF2α Signaling Cascade. Cell Rep. 2015, 10, 684–693. [Google Scholar] [CrossRef]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.; Moonen, C. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, P.; Han, T.; Yu, A.C.; Xu, L. Mechanistic understanding the bioeffects of ultrasound-driven microbubbles to enhance macromolecule delivery. J. Control. Release 2018, 272, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.X.; Guzmán, H.R.; Khan, S.; Prausnitz, M.R. Ultrasound-mediated disruption of cell membranes. I. Quantification of molecular uptake and cell viability. J. Acoust. Soc. Am. 2001, 110, 588–596. [Google Scholar]
- Guzman, H.R.; Nguyen, D.X.; Khan, S.; Prausnitz, M.R. Ultrasound-mediated disruption of cell membranes. II. Heterogeneous effects on cells. J. Acoust. Soc. Am. 2001, 110, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, H.R.; McNamara, A.J.; Nguyen, D.X.; Prausnitz, M.R. Bioeffects caused by changes in acoustic cavitation bubble density and cell concentration: A unified explanation based on cell-to-bubble ratio and blast radius. Ultrasound Med. Biol. 2003, 29, 1211–1222. [Google Scholar] [CrossRef]
- Lammertink, B.H.; Bos, C.; Van Der Wurff-Jacobs, K.M.; Storm, G.; Moonen, C.T.; Deckers, R. Increase of intracellular cisplatin levels and radiosensitization by ultrasound in combination with microbubbles. J. Control. Release 2016, 238, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Karshafian, R.; Bevan, P.D.; Williams, R.; Samac, S.; Burns, P.N. Sonoporation by Ultrasound-Activated Microbubble Contrast Agents: Effect of Acoustic Exposure Parameters on Cell Membrane Permeability and Cell Viability. Ultrasound Med. Biol. 2009, 35, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Hynynen, K. Key Factors That Affect Sonoporation Efficiency in in vitro Settings; The Importance of Standing Wave in Sonoporation. Biochem. Biophys. Res. Commun. 2007, 359, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Chen, X.; Jiang, P.; Wan, J.M.; Qin, P.; Yu, A.C. Induction of Endoplasmic Reticulum Stress by Sonoporation: Linkage to Mitochondria-Mediated Apoptosis Initiation. Ultrasound Med. Biol. 2013, 39, 2382–2392. [Google Scholar] [CrossRef]
- Lejbkowicz, F.; Salzberg, S. Distinct sensitivity of normal and malignant cells to ultrasound in vitro. Environ. Health Perspect. 1997, 105, 1575–1578. [Google Scholar] [CrossRef]
- Trendowski, M.; Wong, V.; Zoino, J.N.; Christen, T.D.; Gadeberg, L.; Sansky, M.; Fondy, T.P. Preferential enlargement of leukemia cells using cytoskeletal-directed agents and cell cycle growth control parameters to induce sensitivity to low frequency ultrasound. Cancer Lett. 2015, 360, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sontum, P.C. Physicochemical Characteristics of Sonazoid™, A New Contrast Agent for Ultrasound Imaging. Ultrasound Med. Biol. 2008, 34, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M. Characteristics of SonoVue (TM). Echocardiography 1999, 16, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Rousseau, S. p38 MAP-Kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambard, J.C.; Lefloch, R.; Pouysségur, J.; Lenormand, P. ERK implication in cell cycle regulation. Biochim. Biophys. Acta 2007, 1773, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ouyang, G.; Bao, S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Mol. Med. 2005, 9, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Rosemblit, C.; A Rivas, M.; Proietti, C.J.; Flaqué, M.C.D.; Mercogliano, M.F.; Beguelin, W.; Maronna, E.; Guzmán, P.; Gercovich, F.G.; et al. p42/p44 MAPK-mediated Stat3Ser727 phosphorylation is required for progestin-induced full activation of Stat3 and breast cancer growth. Endocr. Relat. Cancer 2013, 20, 197–212. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, M.; Oka, M.; Iwasaki, T.; Fukami, Y.; Nishigori, C. Role and Regulation of STAT3 Phosphorylation at Ser727 in Melanocytes and Melanoma Cells. J. Investig. Dermatol. 2012, 132, 1877–1885. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.; Martin, K.J.; Arthur, J.S.C. CREB phosphorylation at Ser133 regulates transcription via distinct mechanisms downstream of cAMP and MAPK signalling. Biochem. J. 2014, 458, 469–479. [Google Scholar] [CrossRef]
- Roux, P.P.; Shahbazian, D.; Vu, H.; Holz, M.K.; Cohen, M.S.; Taunton, J.; Sonenberg, N.; Blenis, J. RAS/ERK Signaling Promotes Site-specific Ribosomal Protein S6 Phosphorylation via RSK and Stimulates Cap-dependent Translation. J. Biol. Chem. 2007, 282, 14056–14064. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Xu, L. Cell experimental studies on sonoporation: State of the art and remaining problems. J. Control. Release 2014, 174, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Trendowski, M. Using the Promise of Sonodynamic Therapy in the Clinical Setting against Disseminated Cancers. Chemother. Res. Prat. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Name | Frequency (MHz) | No. of Cycles | Duty Cycle (%) | Pulse Repetition Frequency (kHz) | MI | Intensity | |
|---|---|---|---|---|---|---|---|
| ISPTA (mW/cm2) | ISPPA (W/cm2) | ||||||
| Low | 1.108 | 4 | 4 | 10 | 0.2 | 74 | 0.66 |
| Medium | 1.108 | 18 | 16 | 10 | 0.3 | 501 | 2.31 |
| High | 1.108 | 41 | 37 | 10 | 0.4 | 2079 | 5.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haugse, R.; Langer, A.; Gullaksen, S.-E.; Sundøy, S.M.; Gjertsen, B.T.; Kotopoulis, S.; McCormack, E. Intracellular Signaling in Key Pathways Is Induced by Treatment with Ultrasound and Microbubbles in a Leukemia Cell Line, but Not in Healthy Peripheral Blood Mononuclear Cells. Pharmaceutics 2019, 11, 319. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11070319
Haugse R, Langer A, Gullaksen S-E, Sundøy SM, Gjertsen BT, Kotopoulis S, McCormack E. Intracellular Signaling in Key Pathways Is Induced by Treatment with Ultrasound and Microbubbles in a Leukemia Cell Line, but Not in Healthy Peripheral Blood Mononuclear Cells. Pharmaceutics. 2019; 11(7):319. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11070319
Chicago/Turabian StyleHaugse, Ragnhild, Anika Langer, Stein-Erik Gullaksen, Silje Maria Sundøy, Bjørn Tore Gjertsen, Spiros Kotopoulis, and Emmet McCormack. 2019. "Intracellular Signaling in Key Pathways Is Induced by Treatment with Ultrasound and Microbubbles in a Leukemia Cell Line, but Not in Healthy Peripheral Blood Mononuclear Cells" Pharmaceutics 11, no. 7: 319. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11070319