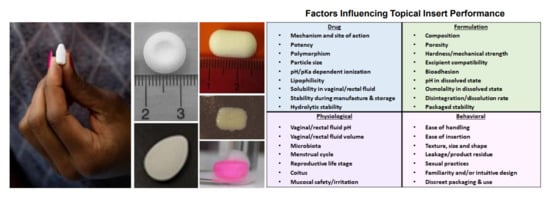
Topical Inserts: A Versatile Delivery Form for HIV Prevention
Abstract
:1. Introduction
2. Overview of Inserts for Female Reproductive Health
2.1. Standardizing the Nomenclature
2.2. Inserts in Development for HIV Prevention
3. Insert Platforms, Technologies and Mechanisms of Dissolution
4. Fast Dissolving Inserts
4.1. Direct Compression Inserts
4.2. Freeze-Dried Inserts
5. Extended-Release Inserts
5.1. Mucoadhesive Matrix-Based
5.2. Osmotic-Controlled
5.3. Integration of Multiparticulate Drug Delivery Systems
6. End-User Acceptability of Inserts for HIV Prevention
7. Considerations for the Development of Inserts for HIV Prevention
7.1. Animal Models
7.2. Incorporating Clinical Studies of Placebo Formulations into Early Product Development
8. Future Directions and Potential Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired Immune Deficiency Syndrome |
| ARV | Antiretroviral |
| CAB | Cabotegravir |
| CDC | Centers for Disease Control and Prevention |
| CG | Carrageenan |
| DCE | Discrete Choice Experiment |
| DMPA | Medroxyprogesterone Acetate |
| DPV | Dapivirine |
| EVG | Elvitegravir |
| EVMS | Eastern Virginia Medical School |
| FTC | Emtricitabine |
| GLP | Good Laboratory Practice |
| GRFT | Griffithsin |
| HIV | Human Immunodeficiency Virus |
| HPMC | Hydroxypropyl Methylcellulose |
| HPV | Human Papilloma Virus |
| HSV | Herpes Simplex Virus |
| IND | Investigational New Drug |
| IPM | International Partnership for Microbicides |
| IVR | Intravaginal Ring |
| LA | Long-acting |
| MPT | Multipurpose Prevention Technologies |
| NHP | Non-human Primate |
| PD | Pharmacodynamics |
| PEP | Post-exposure Prophylaxis |
| PK | Pharmacokinetics |
| PrEP | Pre-exposure Prophylaxis |
| R&D | Research and Development |
| RH | Relative Humidity |
| SHIV | Simian-human Immunodeficiency Virus |
| SIV | Simian Immunodeficiency Virus |
| STI | Sexually Transmitted Infection |
| TAF | Tenofovir Alafenamide Fumarate |
| TDF | Tenofovir Disoproxil Fumarate |
| TFV | Tenofovir |
| UNAIDS | The Joint United Nations Programme on HIV and AIDS |
References
- Joint United Nations Programme on HIV/AIDS. Fact Sheet—Latest Statistics on the Status of the AIDS Epidemic. Available online: http://www.unaids.org/en/resources/fact-sheet (accessed on 24 July 2019).
- UNAIDS. Ambitious Treatment Targets: Writing the Final Chapter of the AIDS Epidemic; UNAIDS: Geneva, Switzerland, 2014. [Google Scholar]
- UNAIDS; Sabin, K. The Prevention Gap Report; UNAIDS: Geneva, Switzerland, 2016. [Google Scholar]
- Baeten, J.M.; Donnell, D.; Ndase, P.; Mugo, N.R.; Campbell, J.D.; Wangisi, J.; Tappero, J.W.; Bukusi, E.A.; Cohen, C.R.; Katabira, E.; et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 2012, 367, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; McMahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapia, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Palanee-Phillips, T.; Brown, E.R.; Schwartz, K.; Soto-Torres, L.E.; Govender, V.; Mgodi, N.M.; Matovu Kiweewa, F.; Nair, G.; Mhlanga, F.; et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N. Engl. J. Med. 2016, 375, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, J.M.; Ramjee, G.; Richardson, B.A.; Gomez, K.; Mgodi, N.; Nair, G.; Palanee, T.; Nakabiito, C.; van der Straten, A.; Noguchi, L.; et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 2015, 372, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, L.; Corneli, A.; Ahmed, K.; Agot, K.; Lombaard, J.; Kapiga, S.; Malahleha, M.; Owino, F.; Manongi, R.; Onyango, J.; et al. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 2012, 367, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Bekker, L.G.; Bukusi, E.; Hellstrm, E.; Kotze, P.; Louw, C.; Martinson, F.; Masenga, G.; Montgomery, E.; Ndaba, N.; et al. Safety, Acceptability and Adherence of Dapivirine Vaginal Ring in a Microbicide Clinical Trial Conducted in Multiple Countries in Sub-Saharan Africa. PLoS ONE 2016, 11, e0147743. [Google Scholar] [CrossRef] [PubMed]
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Delany-Moretlwe, S.; Lombard, C.; Baron, D.; Bekker, L.G.; Nkala, B.; Ahmed, K.; Sebe, M.; Brumskine, W.; Nchabeleng, M.; Palanee-Philips, T.; et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 1241–1250. [Google Scholar] [CrossRef]
- Barrett, S.E.; Teller, R.S.; Forster, S.P.; Li, L.; Mackey, M.A.; Skomski, D.; Yang, Z.; Fillgrove, K.L.; Doto, G.J.; Wood, S.L.; et al. Extended-Duration MK-8591-Eluting Implant as a Candidate for HIV Treatment and Prevention. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Gatto, G. Pharmacokinetics of Tenofovir Alafenamide by Subcutaneous Implant for HIV PrEP. In Proceedings of the CROI, Boston, MA, USA, 4–7 March 2018. [Google Scholar]
- Gunawardana, M.; Remedios-Chan, M.; Miller, C.S.; Fanter, R.; Yang, F.; Marzinke, M.A.; Hendrix, C.W.; Beliveau, M.; Moss, J.A.; Smith, T.J.; et al. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob. Agents Chemother. 2015, 59, 3913–3919. [Google Scholar] [CrossRef]
- Flexner, C. Antiretroviral implants for treatment and prevention of HIV infection. Curr. Opin. HIV AIDS 2018, 13, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, S.; Cardozo, L. The vagina as a route for drug delivery: A review. Int. Urogynecol. J. 2013, 24, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Purohit, T.J.; Hanning, S.M.; Wu, Z. Advances in rectal drug delivery systems. Pharm. Dev. Technol. 2018, 23, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.G.; Zhang, X.; Ganapathi, U.; Szekely, Z.; Flexner, C.W.; Owen, A.; Sinko, P.J. Drug delivery strategies and systems for HIV/AIDS pre-exposure prophylaxis and treatment. J. Control. Release 2015, 219, 669–680. [Google Scholar] [CrossRef] [Green Version]
- USP 35-NF 30. General Chapter <1121> Nomenclature; U.S. Pharmacopeial Convention: Rockville, MD, USA, 2009. [Google Scholar]
- Zydowsky, T.; Friedland, B. Expanding Choices: A Look into the Population Council’s Multipurpose Prevention Technology (MPT) Product Pipeline. Available online: https://www.avac.org/event/expanding-choices (accessed on 25 July 2019).
- Lagenaur, L.A.; Swedek, I.; Lee, P.P.; Parks, T.P. Robust vaginal colonization of macaques with a novel vaginally disintegrating tablet containing a live biotherapeutic product to prevent HIV infection in women. PLoS ONE 2015, 10, e0122730. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.R.; Peet, M.M.; Davis, S.; Doncel, G.F.; Friend, D.R. Evaluation of Rapidly Disintegrating Vaginal Tablets of Tenofovir, Emtricitabine and Their Combination for HIV-1 Prevention. Pharmaceutics 2014, 6, 616–631. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.E.; Clark, M.R.; Friend, D.R.; Garber, D.A.; McNicholl, J.M.; Hendry, R.M.; Doncel, G.F.; Smith, J.M. Pharmacokinetic and safety analyses of tenofovir and tenofovir-emtricitabine vaginal tablets in pigtailed macaques. Antimicrob. Agents Chemother. 2014, 58, 2665–2674. [Google Scholar] [CrossRef]
- Mauck, C.K.; Thurman, A.; Keller, M.; Archer, D.F.; Kimble, T.; Kashuba, A.; Sykes, C.; Friend, D.; Schwartz, J.L.; Doncel, G.F. Pharmacokinetics of Tenofovir and Emtricitabine Delivered by Vaginal Tablets. In Proceedings of the CROI, Seattle, WA, USA, 23–26 February 2015. [Google Scholar]
- Dobard, C.D.; Peet, M.M.; Nishiura, K.; Singh, O.; McCormick, T.; Mitchell, J.; Garcia-Lerma, J.G.; Agrahari, V.; Gupta, P.; Jonnalagadda, S.; et al. Protection Against Vaginal SHIV Infection with an Insert Containing TAF and EVG. In Proceedings of the CROI, Seattle, WA, USA, 4–7 March 2019. [Google Scholar]
- Microbicide Trials Network. Available online: https://mtnstopshiv.org/research/studies/mtn-039 (accessed on 11 June 2019).
- Friend, C.; Steytler, J.; van Niekerk, N.; Nuttall, J.; Devlin, B.; Spence, P.; Derrick, T.; Seaton, E.; Mans, W.; van Tilburg, P.; et al. Safety and Pharmacokinetics of DS003 when Administered to Women as a Vaginal Tablet. In Proceedings of the HIV Research for Prevention (HIVR4P), Madrid, Spain, 22–25 October 2018. [Google Scholar]
- Nuttall, J.; Arien, K.; Michiels, J.; Krit, M.; Vanham, G.; van Tilburg, P.; du Puy, L.; van Niekerk, N.; Nel, A. Pharmacodynamic Activity of DS003, a Novel gp120 Blocker, when Administered to Women as a Vaginal Tablet. In Proceedings of the HIV Research for Prevention (HIVR4P), Madrid, Spain, 22–25 October 2018. [Google Scholar]
- Fu, Y.; Yang, S.; Jeong, S.H.; Kimura, S.; Park, K. Orally fast disintegrating tablets: Developments, technologies, taste-masking and clinical studies. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 433–476. [Google Scholar] [CrossRef]
- Parkash, V.; Maan, S.; Deepika, S.K.Y.; Hemlata, V.J. Fast disintegrating tablets: Opportunity in drug delivery system. J. Adv. Pharm. Technol. Res. 2011, 2, 223–235. [Google Scholar] [CrossRef]
- Zhang, W.; Littlefield, S.; McCormick, T.; Caplena, D.; Masto, E.; Anderson, S.; Linton, K.; Thurman, A.; Schwartz, J.; Clark, M.; et al. Development and Proof-of-Concept Clinical Evaluation of a Freeze-dried Topical Microbicide Insert for On-demand HIV Prevention. In Proceedings of the HIV Research for Prevention (HIVR4P), Chicago, IL, USA, 17–21 October 2016. [Google Scholar]
- Littlefield, S.; Zhang, W.; Schwartz, J.; Clark, M.; Thurman, A.; Archer, D.F.; Mauck, C.K.; Linton, K.; McCormick, T.; Jacot, T.; et al. Clinical Performance, Acceptability, and Optimization of Fast-Dissolve Vaginal Inserts Designed for HIV-Prevention: Results from Two Clinical Studies. In Proceedings of the HIV Research for Prevention (HIVR4P), Chicago, IL, USA, 18 October 2016. [Google Scholar]
- Lal, M.; Lai, M.; Ugaonkar, S.; Wesenberg, A.; Kizima, L.; Rodriguez, A.; Levendosky, K.; Mizenina, O.; Fernandez-Romero, J.; Zydowsky, T. Development of a Vaginal Fast-Dissolving Insert Combining Griffithsin and Carrageenan for Potential Use Against Sexually Transmitted Infections. J. Pharm. Sci. 2018, 107, 2601–2610. [Google Scholar] [CrossRef]
- Lal, M.; Lai, M.; Ugaonkar, S.; Wesenberg, A.; Kizima, L.; Rodriguez, A.; Levendosky, K.; Mizenina, O.; Fernandez-Romero, J.; Zydowsky, T. Self-administered Griffithsin and Carrageenan Containing Microbicide Fast-dissolving Insert as Pre-exposure Prophylaxis Against HIV and HPV Infections. In Proceedings of the HIV Research for Prevention (HIVR4P), Madrid, Spain, 22–25 October 2018. [Google Scholar]
- Derby, N.; Lal, M.; Aravantinou, M.; Kizima, L.; Barnable, P.; Rodriguez, A.; Lai, M.; Wesenberg, A.; Ugaonkar, S.; Levendosky, K.; et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 2018, 9, 3881. [Google Scholar] [CrossRef] [PubMed]
- Merkatz, R.B.; Plagianos, M.; Hoskin, E.; Cooney, M.; Hewett, P.C.; Mensch, B.S. Acceptability of the Nestorone(R)/ethinyl estradiol contraceptive vaginal ring: Development of a model; implications for introduction. Contraception 2014, 90, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.; de la Loge, C.; Abetz, L.; van der Meulen, E.A. The combined contraceptive vaginal ring, NuvaRing: An international study of user acceptability. Contraception 2003, 67, 187–194. [Google Scholar] [CrossRef]
- Roumen, F.J.; Apter, D.; Mulders, T.M.; Dieben, T.O. Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl oestradiol. Hum. Reprod. 2001, 16, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Wieder, D.R.; Pattimakiel, L. Examining the efficacy, safety, and patient acceptability of the combined contraceptive vaginal ring (NuvaRing). Int. J. Womens Health 2010, 2, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Weinrib, R.; Minnis, A.; Agot, K.; Ahmed, K.; Owino, F.; Manenzhe, K.; Cheng, H.; van der Straten, A. End-Users’ Product Preference Across Three Multipurpose Prevention Technology Delivery Forms: Baseline Results from Young Women in Kenya and South Africa. AIDS Behav. 2018, 22, 133–145. [Google Scholar] [CrossRef] [PubMed]
- McConville, C.; Friend, D.R.; Clark, M.R.; Malcolm, K. Preformulation and development of a once-daily sustained-release tenofovir vaginal tablet tablet containing a single excipient. J. Pharm. Sci. 2013, 102, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Cazorla-Luna, R.; Martin-Illana, A.; Notario-Perez, F.; Bedoya, L.M.; Bermejo, P.; Ruiz-Caro, R.; Veiga, M.D. Dapivirine Bioadhesive Vaginal Tablets Based on Natural Polymers for the Prevention of Sexual Transmission of HIV. Polymers 2019, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Cazorla-Luna, R.; Notario-Perez, F.; Martin-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.D. Chitosan-Based Mucoadhesive Vaginal Tablets for Controlled Release of the Anti-HIV Drug Tenofovir. Pharmaceutics 2019, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.B.; Thakur, R.S. Design and evaluation of mucoadhesive vaginal tablets of tenofovir disoproxil fumarate for pre-exposure prophylaxis of HIV. Drug Dev. Ind. Pharm. 2018, 44, 472–483. [Google Scholar] [CrossRef]
- Notario-Perez, F.; Cazorla-Luna, R.; Martin-Illana, A.; Ruiz-Caro, R.; Pena, J.; Veiga, M.D. Tenofovir Hot-Melt Granulation using Gelucire((R)) to Develop Sustained-Release Vaginal Systems for Weekly Protection against Sexual Transmission of HIV. Pharmaceutics 2019, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Notario-Perez, F.; Cazorla-Luna, R.; Martin-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Notario-Perez, F.; Martin-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Pena, J.; Veiga, M.D. Improvement of Tenofovir vaginal release from hydrophilic matrices through drug granulation with hydrophobic polymers. Eur. J. Pharm. Sci. 2018, 117, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.; Teller, R.S.; Mesquita, P.M.; Herold, B.C.; Kiser, P.F. Osmotic pump tablets for delivery of antiretrovirals to the vaginal mucosa. Antivir. Res. 2013, 100, 255–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- das Neves, J.; Amiji, M.M.; Bahia, M.F.; Sarmento, B. Nanotechnology-based systems for the treatment and prevention of HIV/AIDS. Adv. Drug Deliv. Rev. 2010, 62, 458–477. [Google Scholar] [CrossRef]
- das Neves, J.; Nunes, R.; Machado, A.; Sarmento, B. Polymer-based nanocarriers for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Mohideen, M.; Quijano, E.; Song, E.; Deng, Y.; Panse, G.; Zhang, W.; Clark, M.R.; Saltzman, W.M. Degradable bioadhesive nanoparticles for prolonged intravaginal delivery and retention of elvitegravir. Biomaterials 2017, 144, 144–154. [Google Scholar] [CrossRef]
- Whaley, K.J.; Hanes, J.; Shattock, R.; Cone, R.A.; Friend, D.R. Novel approaches to vaginal delivery and safety of microbicides: Biopharmaceuticals, nanoparticles, and vaccines. Antivir. Res. 2010, 88 (Suppl. 1), S55–S66. [Google Scholar] [CrossRef]
- Al-Hashimi, N.; Begg, N.; Alany, R.G.; Hassanin, H.; Elshaer, A. Oral Modified Release Multiple-Unit Particulate Systems: Compressed Pellets, Microparticles and Nanoparticles. Pharmaceutics 2018, 10, 176. [Google Scholar] [CrossRef]
- Xu, M.; Heng, P.W.S.; Liew, C.V. Formulation and process strategies to minimize coat damage for compaction of coated pellets in a rotary tablet press: A mechanistic view. Int. J. Pharm. 2016, 499, 29–37. [Google Scholar] [CrossRef]
- Date, A.A.; Shibata, A.; Goede, M.; Sanford, B.; La Bruzzo, K.; Belshan, M.; Destache, C.J. Development and evaluation of a thermosensitive vaginal gel containing raltegravir+efavirenz loaded nanoparticles for HIV prophylaxis. Antivir. Res. 2012, 96, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Date, A.A.; Shibata, A.; McMullen, E.; La Bruzzo, K.; Bruck, P.; Belshan, M.; Zhou, Y.; Destache, C.J. Thermosensitive Gel Containing Cellulose Acetate Phthalate-Efavirenz Combination Nanoparticles for Prevention of HIV-1 Infection. J. Biomed. Nanotechnol. 2015, 11, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Destache, C.J.; Mandal, S.; Yuan, Z.; Kang, G.; Date, A.A.; Lu, W.; Shibata, A.; Pham, R.; Bruck, P.; Rezich, M.; et al. Topical Tenofovir Disoproxil Fumarate Nanoparticles Prevent HIV-1 Vaginal Transmission in a Humanized Mouse Model. Antimicrob. Agents Chemother. 2016, 60, 3633–3639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Chen, Y.; Gu, K.; Dash, A.; Sayre, C.L.; Davies, N.M.; Ho, E.A. Novel intravaginal nanomedicine for the targeted delivery of saquinavir to CD4+ immune cells. Int. J. Nanomed. 2013, 8, 2847–2858. [Google Scholar] [CrossRef] [Green Version]
- Cunha-Reis, C.; Machado, A.; Barreiros, L.; Araujo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; Segundo, M.A.; Sarmento, B.; das Neves, J. Nanoparticles-in-film for the combined vaginal delivery of anti-HIV microbicide drugs. J. Control. Release 2016, 243, 43–53. [Google Scholar] [CrossRef]
- das Neves, J.; Sarmento, B. Antiretroviral drug-loaded nanoparticles-in-films: A new option for developing vaginal microbicides? Expert Opin. Drug Deliv. 2017, 14, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Cunha-Reis, C.; Araujo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; das Neves, J.; Sarmento, B. Development and in vivo safety assessment of tenofovir-loaded nanoparticles-in-film as a novel vaginal microbicide delivery system. Acta Biomater. 2016, 44, 332–340. [Google Scholar] [CrossRef]
- Montgomery, E. Preference and Choice of Four Vaginally-delivered HIV Prevention Placebo Dosage Forms among Young Southern African Women: Results of the Quatro Randomized Crossover Trial. In Proceedings of the HIV Research for Prevention (HIVR4P), Madrid, Spain, 21–25 October 2018. [Google Scholar]
- Montgomery, E.T.; Beksinska, M.; Mgodi, N.; Schwartz, J.; Weinrib, R.; Browne, E.N.; Mphili, N.; Musara, P.; Jaggernath, M.; Ju, S.; et al. End-user preference for and choice of four vaginally delivered HIV prevention methods among young women in South Africa and Zimbabwe: The Quatro Clinical Crossover Study. J. Int. AIDS Soc. 2019, 22, e25283. [Google Scholar] [CrossRef]
- Nel, A.M.; Mitchnick, L.B.; Risha, P.; Muungo, L.T.M.; Norick, P.M. Acceptability of Vaginal Film, Soft-Gel Capsule, and Tablet as Potential Microbicide Delivery Methods Among African Women. J. Women’s Health 2011, 20, 1207–1214. [Google Scholar] [CrossRef]
- Luecke, E.H.; Cheng, H.; Woeber, K.; Nakyanzi, T.; Mudekunye-Mahaka, I.C.; van der Straten, A.; Team, M.-D.S. Stated product formulation preferences for HIV pre-exposure prophylaxis among women in the VOICE-D (MTN-003D) study. J. Int. AIDS Soc. 2016, 19, 20875. [Google Scholar] [CrossRef]
- Sahoo, C.K.; Nayak, P.K.; Sarangi, D.K.; Sahoo, T.K. Intra Vaginal Drug Delivery System: An Overview. Am. J. Adv. Drug Deliv. 2013, 1, 43–55. [Google Scholar]
- Hatziioannou, T.; Ambrose, Z.; Chung, N.P.; Piatak, M., Jr.; Yuan, F.; Trubey, C.M.; Coalter, V.; Kiser, R.; Schneider, D.; Smedley, J.; et al. A macaque model of HIV-1 infection. Proc. Natl. Acad. Sci. USA 2009, 106, 4425–4429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobard, C.; Sharma, S.; Martin, A.; Pau, C.P.; Holder, A.; Kuklenyik, Z.; Lipscomb, J.; Hanson, D.L.; Smith, J.; Novembre, F.J.; et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J. Virol. 2012, 86, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, S.A.; Guenthner, P.C.; Lin, C.Y.; Dobard, C.; Sharma, S.; Adams, D.R.; Otten, R.A.; Heneine, W.; Hendry, R.M.; McNicholl, J.M.; et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J. Acquir. Immune Defic. Syndr. 2011, 57, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Veazey, R.S.; Shattock, R.J.; Klasse, P.J.; Moore, J.P. Animal models for microbicide studies. Curr. HIV Res. 2012, 10, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Morrow, W.J.; Wharton, M.; Lau, D.; Levy, J.A. Small animals are not susceptible to human immunodeficiency virus infection. J. Gen. Virol. 1987, 68, 2253–2257. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, P.; Jackson, M.C.; Millman, N.; Sobrero, A.J. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. J. Reprod. Fertil. 1969, 20, 85–93. [Google Scholar] [CrossRef]
- Doncel, G.F.; Chandra, N.; Fichorova, R.N. Preclinical assessment of the proinflammatory potential of microbicide candidates. J. Acquir. Immune Defic. Syndr. 2004, 37 (Suppl. 3), S174–S180. [Google Scholar] [CrossRef]
- Denton, P.W.; Garcia, J.V. Humanized mouse models of HIV infection. AIDS Rev. 2011, 13, 135–148. [Google Scholar]




| Example Products | Indication |
|---|---|
| Mycelex-G | Treat vulvovaginal yeast (Candida) infections |
| Semicid, Encare® | Prevent pregnancy |
| Intrarosa®, Imvexxy® | Treat moderate-to-severe dyspareunia due to menopause |
| Endometrin® | Support embryo implantation in early pregnancy |
| Vagifem® | Treat vaginal irritation and dryness caused by menopause |
| Product Name | Developer | Insert Technology | Indication | Development Phase |
|---|---|---|---|---|
| Tenofovir Alafenamide Fumarate/Elvitegravir Topical Insert | CONRAD/EVMS (Arlington and Norfolk, VA, USA) | Compressed | HIV, HSV (vaginal or rectal use) | Phase I |
| Griffithsin/Carrageenan Fast Dissolve Insert (PC-9500) | Population Council (New York, NY, USA) in collaboration with PATH (Seattle, WA, USA) | Freeze-Dried | HIV, HPV, HSV (vaginal use) | Preclinical * |
| DS003 Vaginal Tablet | IPM (Silver Spring, MD, USA) | Compressed | HIV (vaginal use) | Phase I |
| MucoCept® Lactobacillus Vaginal Tablet | Osel (Mountain View, CA, USA) | Freeze-Dried | HIV (vaginal use) | Preclinical |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peet, M.M.; Agrahari, V.; Anderson, S.M.; Hanif, H.; Singh, O.N.; Thurman, A.R.; Doncel, G.F.; Clark, M.R. Topical Inserts: A Versatile Delivery Form for HIV Prevention. Pharmaceutics 2019, 11, 374. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080374
Peet MM, Agrahari V, Anderson SM, Hanif H, Singh ON, Thurman AR, Doncel GF, Clark MR. Topical Inserts: A Versatile Delivery Form for HIV Prevention. Pharmaceutics. 2019; 11(8):374. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080374
Chicago/Turabian StylePeet, M. Melissa, Vivek Agrahari, Sharon M. Anderson, Homaira Hanif, Onkar N. Singh, Andrea R. Thurman, Gustavo F. Doncel, and Meredith R. Clark. 2019. "Topical Inserts: A Versatile Delivery Form for HIV Prevention" Pharmaceutics 11, no. 8: 374. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11080374