Effect of Size and Concentration of PLGA-PEG Nanoparticles on Activation and Aggregation of Washed Human Platelets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Characterisation of PLGA-PEG NPs
2.2.2. Preparation of Washed Platelets
2.2.3. Effect of Size and Concentration of NPs on Platelet Aggregation
2.2.4. Effect of Size and Concentration of NPs on Platelet Activation
2.2.5. Confocal Microscopy of the Effect of Incubation Time on The Interaction of PLGA-PEG NPs with Washed Platelets
2.2.6. Statistical Analysis
3. Results
3.1. Characterisation of NPs Formulated
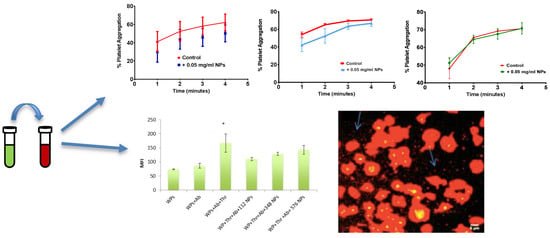
3.2. Effect of Size and Concentration of NPs on Platelet Aggregation
3.3. Effect of Size of NPs on Platelet Activation Profile
3.4. Effect of size of NPs on Thrombin Activation of Washed Platelets
3.5. Confocal Microscopy of the Effect of Incubation Time on the Interaction of NPs and Washed Platelets
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nandagiri, V.K.; Gentile, P.; Chiono, V.; Tonda-Turo, C.; Matsiko, A.; Ramtoola, Z.; Montevecchi, F.M.; Ciardelli, G. Incorporation of PLGA nanoparticles into porous chitosan–gelatin scaffolds: Influence on the physical properties and cell behavior. J. Mech. Behav. Biomed. Mater. 2011, 4, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Patrick, C. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Mohan, L.J.; Daly, J.S.; Ryan, B.M.; Ramtoola, Z. The future of nanomedicine in optimising the treatment of inflammatory bowel disease. Scand. J. Gastroenterol. 2019, 54, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jiang, L.; Wang, Y.; Gang, F.; Xu, N.; Li, T.; Liu, Z.; Chi, Y.; Wang, X.; Zhao, L.; et al. 3D Printing of Conductive Tissue Engineering Scaffolds Containing Polypyrrole Nanoparticles with Different Morphologies and Concentrations. Mater. 2019, 12, 2491. [Google Scholar] [CrossRef]
- Du, J.; Sun, Y.; Shi, Q.-S.; Liu, P.-F.; Zhu, M.-J.; Wang, C.-H.; Du, L.-F.; Duan, Y.-R. Biodegradable Nanoparticles of mPEG-PLGA-PLL Triblock Copolymers as Novel Non-Viral Vectors for Improving siRNA Delivery and Gene Silencing. Int. J. Mol. Sci. 2012, 13, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.-W.; Langer, R.; Farokhzad, O.C. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomater. 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Mattu, C.; Pabari, R.; Boffito, M.; Sartori, S.; Ciardelli, G.; Ramtoola, Z.; Pabari, R. Comparative evaluation of novel biodegradable nanoparticles for the drug targeting to breast cancer cells. Eur. J. Pharm. Biopharm. 2013, 85, 463–472. [Google Scholar] [CrossRef]
- Kirby, B.P.; Pabari, R.; Al Baharna, M.; Walsh, J.; Ramtoola, Z.; Chen, C.-N.; Chen, C. Comparative evaluation of the degree of pegylation of poly (lactic-co-glycolic acid) nanoparticles in enhancing central nervous system delivery of loperamide. J. Pharm. Pharmacol. 2013, 65, 1473–1481. [Google Scholar] [CrossRef]
- O’Donnell, A.; Moollan, A.; Baneham, S.; Ozgul, M.; Pabari, R.M.; Cox, D.; Kirby, B.P.; Ramtoola, Z. Intranasal and intravenous administration of octa-arginine modified poly (lactic-co-glycolic acid) nanoparticles facilitates central nervous system delivery of loperamide. J. Pharm. Pharmacol. 2015, 67, 525–536. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Yan, B. Enabling Anticancer Therapeutics by Nanoparticle Carriers: The Delivery of Paclitaxel. Int. J. Mol. Sci. 2011, 12, 4395–4413. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Modery-Pawlowski, C.L.; Menegatti, S.; Kumar, S.; Vogus, D.R.; Tian, L.L.; Chen, M.; Squires, T.M.; Gupta, A.S.; Mitragotri, S. Platelet-like nanoparticles: Mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano 2014, 8, 11243–11253. [Google Scholar] [CrossRef] [PubMed]
- Serda, R.E.; Godin, B.; Blanco, E.; Chiappini, C.; Ferrari, M. Multi-stage delivery nano-particle systems for therapeutic applications. Biochim. Biophys. Acta, Gen. Subj. 2011, 1810, 317–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özcan, I.; Segura-Sanchez, F.; Bouchemal, K.; Sezak, M.; Özer, Ö.; Güneri, T.; Ponchel, G. Pegylation of poly (γ-benzyl-L-glutamate) nanoparticles is efficient for avoiding mononuclear phagocyte system capture in rats. Int. J. Nanomed. 2010, 5, 1103. [Google Scholar] [CrossRef]
- Santos-Martinez, M.J.; Inkielewicz-Stępniak, I.; Medina, C.; Rahme, K.; D’Arcy, D.M.; Fox, D.; Holmes, J.D.; Zhang, H.; Radomski, M.W. The use of quartz crystal microbalance with dissipation (QCM-D) for studying nanoparticle-induced platelet aggregation. Int. J. Nanomed. 2012, 7, 243–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; McNeil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomed. Nanotechnol. Bio. Med. 2009, 5, 106–117. [Google Scholar] [CrossRef]
- Cheng, F.-Y.; Wang, S.P.-H.; Su, C.-H.; Tsai, T.-L.; Wu, P.-C.; Shieh, D.-B.; Chen, J.-H.; Hsieh, P.C.-H.; Yeh, C.-S. Stabilizer-free poly(lactide-co-glycolide) nanoparticles for multimodal biomedical probes. Biomater. 2008, 29, 2104–2112. [Google Scholar] [CrossRef] [PubMed]
- Koziara, J.M.; Oh, J.J.; Akers, W.S.; Ferraris, S.P.; Mumper, R.J. Blood Compatibility of Cetyl Alcohol/Polysorbate-Based Nanoparticles. Pharm. Res. 2005, 22, 1821–1828. [Google Scholar] [CrossRef]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar] [CrossRef] [Green Version]
- Ramtoola, Z.; Lyons, P.; Keohane, K.; Kerrigan, S.W.; Kirby, B.P.; Kelly, J.G. Investigation of the interaction of biodegradable micro-and nanoparticulate drug delivery systems with platelets. J. Pharm. Pharmacol. 2011, 63, 26–32. [Google Scholar] [CrossRef]
- Corbalan, J.J.; Medina, C.; Jacoby, A.; Malinski, T.; Radomski, M.W. Amorphous silica nanoparticles aggregate human platelets: Potential implications for vascular homeostasis. Int. J. Nanomed. 2012, 7, 631–639. [Google Scholar]
- Shrivastava, S.; Bera, T.; Singh, S.K.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of Antiplatelet Properties of Silver Nanoparticles. ACS Nano 2009, 3, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- MMiller, V.M.; Hunter, L.W.; Chu, K.; Kaul, V.; Squillace, P.D.; Lieske, J.C.; Jayachandran, M. Biologic nanoparticles and platelet reactivity. Nanomed. 2009, 4, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabari, R.M.; Mattu, C.; Partheeban, S.; Almarhoon, A.; Boffito, M.; Ciardelli, G.; Ramtoola, Z. Novel polyurethane-based nanoparticles of infliximab to reduce inflammation in an in-vitro intestinal epithelial barrier model. Int. J. Pharm. 2019, 565, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Fornaguera, C.; Solans, C. Characterization of Polymeric Nanoparticle Dispersions for Biomedical Applications: Size, Surface Charge and Stability. Pharm. Nanotechnol. 2018, 6, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Schmaier, A.H. Platelet aggregation testing in platelet-rich plasma: Description of procedures with the aim to develop standards in the field. Am. J. Clin. Pathol. 2005, 123, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Ilinskaya, A.N.; A Dobrovolskaia, M. Nanoparticles and the blood coagulation system. Part I: Benefits of nanotechnology. Nanomed. 2013, 8, 773–784. [Google Scholar] [CrossRef]
- Ragaseema, V.; Unnikrishnan, S.; Krishnan, V.K.; Krishnan, L.K. The antithrombotic and antimicrobial properties of PEG-protected silver nanoparticle coated surfaces. Biomater. 2012, 33, 3083–3092. [Google Scholar] [CrossRef]
- Mayer, A.; Vadon, M.; Rinner, B.; Novak, A.; Wintersteiger, R.; Fröhlich, E. The role of nanoparticle size in hemocompatibility. Toxicol. 2009, 258, 139–147. [Google Scholar] [CrossRef]
- Gupalo, E.; Kuk, C.; Qadura, M.; Buriachkovskaia, L.; Othman, M. Platelet–adenovirus vs. inert particles interaction: Effect on aggregation and the role of platelet membrane receptors. Platelets 2013, 24, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Jiang, X.; Yang, L.; Landfester, K.; Mailänder, V.; Simmet, T.; Nienhaus, G.U. Nanoparticle interactions with live cells: Quantitative fluorescence microscopy of nanoparticle size effects. Beilstein J. Nanotechnol. 2014, 5, 2388–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranic, S.; Boggetto, N.; Contremoulins, V.; Mornet, S.; Reinhardt, N.; Marano, F.; Baeza-Squiban, A.; Boland, S. Deciphering the mechanisms of cellular uptake of engineered nanoparticles by accurate evaluation of internalization using imaging flow cytometry. Part. Fibre Toxicol. 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.P.; Santos-Martinez, M.J.; Medina, C.; Jain, N.; Radomski, M.W.; Prina-Mello, A.; Volkov, Y. CdTe quantum dots induce activation of human platelets: Implications for nanoparticle hemocompatibility. Int. J. Nanomed. 2015, 10, 2723–2734. [Google Scholar]






| PLGA-PEG (mg/mL) | PS (nm) | PDI | ZP (mV) |
|---|---|---|---|
| 10 | 111.55 ± 5.81 | 0.10 ± 0.02 | −22.20 ± 5.71 |
| 55 | 348.00 ± 23.61 | 0.54 ± 0.04 | −12.40 ± 6.30 |
| 100 | 576.19 ± 6.82 | 0.70 ± 0.06 | −9.50 ± 14.56 |
| PLGA-PEG NPs (mg/mL) | % PA (112 nm) | % PA (348 nm) | % PA (576 nm) |
|---|---|---|---|
| 0 (Control) | 72.75 ± 1.89 | 74.75 ± 1.93 | 72.25 ± 0.62 |
| 0.05 | 72.00 ± 0.91 | 73.50 ± 1.88 | 75.00 ± 4.63 |
| 0.1 | 73.00 ± 1.23 | 76.20 ± 2.99 | 69.75 ± 8.22 |
| 0.25 | 69.00 ± 1.68 | 66.00 ± 5.11 | 74.00 ± 4.89 |
| 0.5 | 73.50 ± 0.96 | 70.25 ± 4.13 | 68.00 ± 4.97 |
| 1 | 72.75 ± 0.85 | 62.50 ± 3.59 | 71.25 ± 5.27 |
| 1.5 | 72.25 ± 1.55 | 28.40 ± 3.47*** | 68.75 ± 2.10 |
| 2.2 | 71.00 ± 1.80 | 27.00 ± 3.10*** | 49.00 ± 5.18* |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhaidar, R.; Green, J.; Alfahad, K.; Samanani, S.; Moollan, N.; O’Neill, S.; Ramtoola, Z. Effect of Size and Concentration of PLGA-PEG Nanoparticles on Activation and Aggregation of Washed Human Platelets. Pharmaceutics 2019, 11, 514. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11100514
Bakhaidar R, Green J, Alfahad K, Samanani S, Moollan N, O’Neill S, Ramtoola Z. Effect of Size and Concentration of PLGA-PEG Nanoparticles on Activation and Aggregation of Washed Human Platelets. Pharmaceutics. 2019; 11(10):514. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11100514
Chicago/Turabian StyleBakhaidar, Rana, Joshua Green, Khaled Alfahad, Shazia Samanani, Nabeehah Moollan, Sarah O’Neill, and Zebunnissa Ramtoola. 2019. "Effect of Size and Concentration of PLGA-PEG Nanoparticles on Activation and Aggregation of Washed Human Platelets" Pharmaceutics 11, no. 10: 514. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics11100514