Distribution of Gold Nanoparticles in the Anterior Chamber of the Eye after Intracameral Injection for Glaucoma Therapy
Abstract
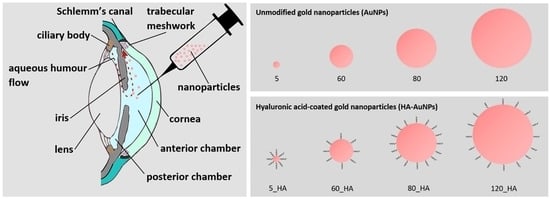
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Preparation of HA-Modified AuNPs
2.4. Characterization of Gold NPs
2.5. Colloidal Stability of Gold NPs
2.6. Uptake of Gold NPs in Different Cell Types In Vitro
2.7. Perfusion Experiments of Porcine Eyes Ex Vivo
2.8. Electron Microscopy Examination of the Trabecular Meshwork
2.9. Gold Content of the Cells and Tissue Samples
2.10. Statistical Analysis
3. Results
3.1. Modification and Physicochemical Characterization of Gold NPs
3.2. Colloidal Stability of Gold NPs
3.3. Perfusion of Porcine Eyes with Gold NPs Ex Vivo
3.4. Distribution Pattern of Gold NPs in the Outflow Tissue
3.5. Cellular Uptake of Gold NPs In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Kapetanakis, V.V.; Chan, M.P.Y.; Foster, P.J.; Cook, D.G.; Owen, C.G.; Rudnicka, A.R. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): A systematic review and meta-analysis. Br. J. Ophthalmol. 2015, 100, 86–93. [Google Scholar] [CrossRef]
- Jünemann, A.G.; Chorągiewicz, T.; Ozimek, M.; Grieb, P.; Rejdak, R. Drug bioavailability from topically applied ocular drops. Does drop size matter? Ophthalmol. J. 2016, 1, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Mietzner, R.; Breunig, M. Causative glaucoma treatment: Promising targets and delivery systems. Drug Discov. Today 2019, 24, 1606–1613. [Google Scholar] [CrossRef]
- Honjo, M.; Tanihara, H. Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Jpn. J. Ophthalmol. 2018, 62, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Fingeret, M.; Gaddie, I.B.; Bloomenstein, M. Latanoprostene bunod ophthalmic solution 0.024%: A new treatment option for open-angle glaucoma and ocular hypertension. Clin. Exp. Optom. 2019, 102, 541–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guter, M. Layer-By-Layer Nanoparticles for Glaucoma Therapy. Ph.D. Thesis, Universität Regensburg, Regensburg, Germany, 2018. [Google Scholar]
- Cassidy, P.S.; Kelly, R.A.; Reina-Torres, E.; Sherwood, J.M.; Humphries, M.M.; Kiang, A.-S.; Farrar, G.J.; O’Brien, C.; Campbell, M.; Stamer, W.D.; et al. siRNA targeting Schlemm’s canal endothelial tight junctions enhances outflow facility and reduces IOP in a steroid-induced OHT rodent model. Mol. Ther. Methods Clin. Dev. 2021, 20, 86–94. [Google Scholar] [CrossRef]
- Baran-Rachwalska, P.; Torabi-Pour, N.; Sutera, F.M.; Ahmed, M.; Thomas, K.; Nesbit, M.A.; Welsh, M.; Moore, C.T.; Saffie-Siebert, S.R. Topical siRNA delivery to the cornea and anterior eye by hybrid silicon-lipid nanoparticles. J. Control. Release 2020, 326, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Dillinger, A.E.; Guter, M.; Froemel, F.; Weber, G.R.; Perkumas, K.; Stamer, W.D.; Ohlmann, A.; Fuchshofer, R.; Breunig, M. Intracameral Delivery of Layer-by-Layer Coated siRNA Nanoparticles for Glaucoma Therapy. Small 2018, 14, e1803239. [Google Scholar] [CrossRef] [PubMed]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef]
- Breunig, M.; Babl, S.; Liebl, R.; Guter, M. Layer-by-layer coated nanoparticles for glaucoma therapy: Focusing on the transport and cellular uptake in the trabecular meshwork. Acta Ophthalmol. 2016, 94, 94. [Google Scholar] [CrossRef]
- Braunger, B.M.; Fuchshofer, R.; Tamm, E.R. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015, 95, 173–181. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Kashaw, S.K.; Iyer, A.K. siRNA Delivery Strategies: A Comprehensive Review of Recent Developments. Nanomaterials 2017, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Lee, C.-H.; Chen, S.-T.; Lai, J.-Y.; Wu, K.C.-W. Gelatin-functionalized mesoporous silica nanoparticles with sustained release properties for intracameral pharmacotherapy of glaucoma. J. Mater. Chem. B 2017, 5, 7008–7013. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Tabish, T.A.; Dey, P.; Mosca, S.; Salimi, M.; Palombo, F.; Matousek, P.; Stone, N. Smart Gold Nanostructures for Light Mediated Cancer Theranostics: Combining Optical Diagnostics with Photothermal Therapy. Adv. Sci. 2020, 7, 1903441. [Google Scholar] [CrossRef]
- Apaolaza, P.; Busch, M.; Asin-Prieto, E.; Peynshaert, K.; Rathod, R.; Remaut, K.; Dünker, N.; Göpferich, A. Hyaluronic acid coating of gold nanoparticles for intraocular drug delivery: Evaluation of the surface properties and effect on their distribution. Exp. Eye Res. 2020, 198, 108151. [Google Scholar] [CrossRef]
- Guter, M.; Breunig, M. Hyaluronan as a promising excipient for ocular drug delivery. Eur. J. Pharm. Biopharm. 2017, 113, 34–49. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Yang, J.-A.; Jung, H.S.; Beack, S.; Choi, J.E.; Hur, W.; Koo, H.; Kim, K.; Yoon, S.K.; Hahn, S.K. Hyaluronic Acid–Gold Nanoparticle/Interferon α Complex for Targeted Treatment of Hepatitis C Virus Infection. ACS Nano 2012, 6, 9522–9531. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron-microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Wang, W.; Wei, Q.-Q.; Wang, J.; Wang, B.-C.; Zhang, S.-H.; Yuan, Z. Role of thiol-containing polyethylene glycol (thiol-PEG) in the modification process of gold nanoparticles (AuNPs): Stabilizer or coagulant? J. Colloid Interface Sci. 2013, 404, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Allingham, R.R.; de Kater, A.W.; Ethier, R.C. Schlemm’s Canal and Primary Open Angle Glaucoma: Correlation Between Schlemn’s Canal Dimensions and Outflow Facility. Exp. Eye Res. 1996, 62, 101–109. [Google Scholar] [CrossRef]
- Ashpole, N.E.; Overby, D.R.; Ethier, C.R.; Stamer, W.D. Shear Stress-Triggered Nitric Oxide Release from Schlemm’s Canal Cells. Investig. Opthalmol. Vis. Sci. 2014, 55, 8067–8076. [Google Scholar] [CrossRef]
- McDonnell, F.; Perkumas, K.M.; Ashpole, N.E.; Kalnitsky, J.; Sherwood, J.M.; Overby, D.R.; Stamer, W.D. Shear Stress in Schlemm’s Canal as a Sensor of Intraocular Pressure. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef]
- Occhiutto, M.L.; Maranhão, R.C.; Costa, V.P.; Konstas, A.G. Nanotechnology for Medical and Surgical Glaucoma Therapy—A Review. Adv. Ther. 2019, 37, 155–199. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Ann. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater. 2019, 31, e1805730. [Google Scholar] [CrossRef] [PubMed]
- Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.; Lee, K.H.; Pomper, M.G.; Searson, P.C. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J. Control. Release 2014, 187, 133–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, T.A.; Joshi, P.P.; Sokolov, K. Preventing Protein Adsorption and Macrophage Uptake of Gold Nanoparticlesviaa Hydrophobic Shield. ACS Nano 2012, 6, 9182–9190. [Google Scholar] [CrossRef] [Green Version]
- Barreto, Â.; Luis, L.G.; Girão, A.V.; Trindade, T.; Soares, A.; Oliveira, M. Behavior of colloidal gold nanoparticles in different ionic strength media. J. Nanoparticle Res. 2015, 17, 1–13. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Millard, C.B.; Tripathi, B.J. Protein composition of human aqueous humor: SDS-PAGE analysis of surgical and post-mortem samples. Exp. Eye Res. 1989, 48, 117–130. [Google Scholar] [CrossRef]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene glycol functionalized gold nanoparticles: The influence of capping density on stability in various media. Gold Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]
- Lin, S.; Ge, C.; Wang, D.; Xie, Q.; Wu, B.; Wang, J.; Nan, K.; Zheng, Q.; Chen, W. Overcoming the Anatomical and Physiological Barriers in Topical Eye Surface Medication Using a Peptide-Decorated Polymeric Micelle. ACS Appl. Mater. Interfaces 2019, 11, 39603–39612. [Google Scholar] [CrossRef]
- Swetledge, S.; Jung, J.P.; Carter, R.; Sabliov, C. Distribution of polymeric nanoparticles in the eye: Implications in ocular disease therapy. J. Nanobiotechnol. 2021, 19, 1–19. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous Humor Dynamics: A Review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [Green Version]
- del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Tojo, K.J.; Ohtori, A. Pharmacokinetic model of intravitreal drug injection. Math. Biosci. 1994, 123, 59–75. [Google Scholar] [CrossRef]
- Overby, D.R.; Zhou, E.H.; Vargas-Pinto, R.; Pedrigi, R.M.; Fuchshofer, R.; Braakman, S.T.; Gupta, R.; Perkumas, K.M.; Sherwood, J.M.; Vahabikashi, A.; et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 13876–13881. [Google Scholar] [CrossRef] [Green Version]
- Vingolo, E.M.; Chabib, A.; Anselmucci, F. Regeneration of trabecular meshwork in primary open angle glaucoma by stem cell therapy: A new treatment approach. Transpl. Res. Risk Manag. 2019, 11, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Liebmann, J.M.; Barton, K.; Weinreb, R.N.; Eichenbaum, D.A.; Gupta, P.K.; McCabe, C.M.; Wolfe, J.D.; Ahmed, I.; Sheybani, A.; Craven, E.R. Evolving Guidelines for Intracameral Injection. J. Glaucoma 2020, 29, S1–S7. [Google Scholar] [CrossRef]
- Shah, T.J.; Conway, M.D.; Peyman, G.A. Intracameral dexamethasone injection in the treatment of cataract surgery induced inflammation: Design, development, and place in therapy. Clin. Ophthalmol. 2018, 12, 2223–2235. [Google Scholar] [CrossRef] [Green Version]
- Braga-Mele, R.; Chang, D.F.; Henderson, B.A.; Mamalis, N.; Talley-Rostov, A.; Vasavada, A. Intracameral antibiotics: Safety, efficacy, and preparation. J. Cataract. Refract. Surg. 2014, 40, 2134–2142. [Google Scholar] [CrossRef]
- Mietzner, R.; Kade, C.; Froemel, F.; Pauly, D.; Stamer, W.D.; Ohlmann, A.; Wegener, J.; Fuchshofer, R.; Breunig, M. Fasudil Loaded PLGA Microspheres as Potential Intravitreal Depot Formulation for Glaucoma Therapy. Pharmaceutics 2020, 12, 706. [Google Scholar] [CrossRef]







| Criteria | 60 nm | 120 nm | Statistics |
|---|---|---|---|
| particles per 1000 µm² –of total TM (±SEM) –of outer TM (±SEM) –of inner TM (±SEM) | 1.18 ± 0.75 1.55 ± 1.12 1.02 ± 0.14 | 1.71 ± 0.62 1.05 ± 0.61 2.12 ± 0.87 | p = 0.61 p = 0.66 p = 0.37 |
| aggregated NPs | 0 of 125 | 2 of 73 |
| Tissue/Condition | Immortalized Cell Lines | Primary Cells |
|---|---|---|
| trabecular meshwork | HTM-N | hTM (no. 134 and 136) |
| endothelial cells | – | SC (no. 74 and 79) HUVEC |
| disease model | – | fibroblasts |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonntag, T.; Froemel, F.; Stamer, W.D.; Ohlmann, A.; Fuchshofer, R.; Breunig, M. Distribution of Gold Nanoparticles in the Anterior Chamber of the Eye after Intracameral Injection for Glaucoma Therapy. Pharmaceutics 2021, 13, 901. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13060901
Sonntag T, Froemel F, Stamer WD, Ohlmann A, Fuchshofer R, Breunig M. Distribution of Gold Nanoparticles in the Anterior Chamber of the Eye after Intracameral Injection for Glaucoma Therapy. Pharmaceutics. 2021; 13(6):901. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13060901
Chicago/Turabian StyleSonntag, Tobias, Franziska Froemel, W. Daniel Stamer, Andreas Ohlmann, Rudolf Fuchshofer, and Miriam Breunig. 2021. "Distribution of Gold Nanoparticles in the Anterior Chamber of the Eye after Intracameral Injection for Glaucoma Therapy" Pharmaceutics 13, no. 6: 901. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13060901