Multifunctional Nanofibrous Dressing with Antimicrobial and Anti-Inflammatory Properties Prepared by Needle-Free Electrospinning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanofiber Preparation
2.2.1. Polymer Solution Preparation
2.2.2. Solution Characterisation
2.2.3. Electrospinning of Polymer Solutions
2.3. Nanofiber Characterisation
2.3.1. Morphology and Fiber Diameter
2.3.2. Tensile Properties
2.3.3. Swelling Index
- WI: Initial weight specimen
- WA: Weight of specimen after swelling
2.3.4. In Vitro Drug Release
2.4. Biological Tests
2.4.1. In Vitro Cytotoxicity
- AS: Absorption of the samples
- AC: Absorption of the control
2.4.2. Antimicrobial Activity Testing Using the Disc Diffusion Assay
2.4.3. Anti-Inflammatory Activity Testing
- AS: Absorption of the samples
- AC: Absorption of the control
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Polymer Solution Properties on Nanofiber Morphology
3.2. Mechanical Properties of Nanofibers
3.3. Swelling Properties of Nanofibers
3.4. In Vitro Release of Chloramphenicol
3.5. Cytotoxicity Testing
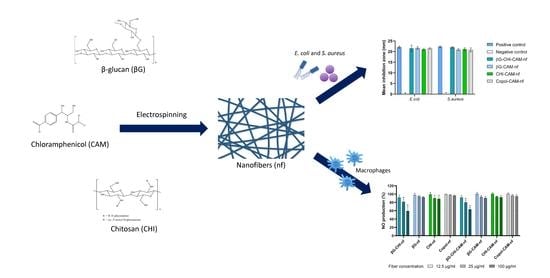
3.6. Antimicrobial Activity
3.7. Anti-Inflammatory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sganga, G.; Pea, F.; Aloj, D.; Corcione, S.; Pierangeli, M.; Stefani, S.; Rossolini, G.M.; Menichetti, F. Acute wound infections management: The ’Don’ts’ from a multidisciplinary expert panel. Expert Rev. Anti-Infect. Ther. 2020, 18, 231–240. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Lorenzo, D. Chloramphenicol Resurrected: A Journey from Antibiotic Resistance in Eye Infections to Biofilm and Ocular Microbiota. Microorganisms 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Grammatikos, A.P.; Michalopoulos, A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev. Anti-Infect. Ther. 2008, 6, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Andaluz-Scher, L.; Medow, N.B. Chloramphenicol Eye Drops: An Old Dog in a New House. Ophthalmology 2020, 127, 1289–1291. [Google Scholar] [CrossRef]
- Takada, S.; Fujiwara, S.; Inoue, T.; Kataoka, Y.; Hadano, Y.; Matsumoto, K.; Morino, K.; Shimizu, T. Meningococcemia in Adults: A Review of the Literature. Intern. Med. 2016, 55, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, E.B.; Ribeiro, A.F.; Ferreira, M.I.; Teixeira, M.C.; Shimojo, A.A.M.; Soriano, J.L.; Naveros, B.C.; Durazzo, A.; Lucarini, M.; Souto, S.B.; et al. New Nanotechnologies for the Treatment and Repair of Skin Burns Infections. Int. J. Mol. Sci. 2020, 21, 393. [Google Scholar] [CrossRef] [Green Version]
- Maleki Dizaj, S.; Sharifi, S.; Jahangiri, A. Electrospun nanofibers as versatile platform in antimicrobial delivery: Current state and perspectives. Pharm. Dev. Technol. 2019, 24, 1187–1199. [Google Scholar] [CrossRef]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef] [PubMed]
- Dubsky, M.; Kubinova, S.; Sirc, J.; Voska, L.; Zajicek, R.; Zajicova, A.; Lesny, P.; Jirkovska, A.; Michalek, J.; Munzarova, M.; et al. Nanofibers prepared by needleless electrospinning technology as scaffolds for wound healing. J. Mater. Sci. Mater. Med. 2012, 23, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Pelipenko, J.; Kocbek, P.; Govedarica, B.; Rosic, R.; Baumgartner, S.; Kristl, J. The topography of electrospun nanofibers and its impact on the growth and mobility of keratinocytes. Eur. J. Pharm. Biopharm. 2013, 84, 401–411. [Google Scholar] [CrossRef]
- Doostmohammadi, M.; Forootanfar, H.; Ramakrishna, S. Regenerative medicine and drug delivery: Progress via electrospun biomaterials. Mater. Sci. Eng. C 2020, 109, 110521. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Geng, Y.; Zhou, F.; Williams, G.R. Developing and scaling up fast-dissolving electrospun formulations based on poly(vinylpyrrolidone) and ketoprofen. J. Drug Deliv. Sci. Technol. 2021, 61, 102138. [Google Scholar] [CrossRef]
- Quan, Z.; Wang, Y.; Zu, Y.; Qin, X.; Yu, J. A rotary spinneret for high output of electrospun fibers with bimodal distribution. Eur. Polym. J. 2021, 159, 110707. [Google Scholar] [CrossRef]
- Vass, P.; Szabo, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Demuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-up of electrospinning technology: Applications in the pharmaceutical industry. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar, K.; Nagy, Z.K. Corona-electrospinning: Needleless method for high-throughput continuous nanofiber production. Eur. Polym. J. 2016, 74, 279–286. [Google Scholar] [CrossRef]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef]
- Yalcinkaya, F. Preparation of various nanofiber layers using wire electrospinning system. Arab. J. Chem. 2017, 12, 5162–5172. [Google Scholar] [CrossRef]
- Grip, J.; Engstad, R.E.; Skjaeveland, I.; Škalko-Basnet, N.; Isaksson, J.; Basnet, P.; Holsæter, A.M. Beta-glucan-loaded nanofiber dressing improves wound healing in diabetic mice. Eur. J. Pharm. Sci. 2018, 121, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Xu, Z.; Wen, X.; Wei, C. A nano chitosan membrane barrier prepared via Nanospider technology with non-toxic solvent for peritoneal adhesions’ prevention. J. Biomater. Appl. 2021, 36, 321–331. [Google Scholar] [CrossRef] [PubMed]
- El-Newehy, M.H.; Al-Deyab, S.S.; Kenawy, E.-R.; Abdel-Megeed, A. Nanospider Technology for the Production of Nylon-6 Nanofibers for Biomedical Applications. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A Mini-Review: Needleless Electrospinning of Nanofibers for Pharmaceutical and Biomedical Applications. Processes 2020, 8, 673. [Google Scholar] [CrossRef]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Li, Y.; Li, P.; Kong, L. Functional Nanofibrous Biomaterials of Tailored Structures for Drug Delivery-A Critical Review. Pharmaceutics 2020, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; Kingshott, P.; McArthur, S.L. Electrospun Polystyrene Fiber Diameter Influencing Bacterial Attachment, Proliferation, and Growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing-A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [Green Version]
- Majtan, J.; Jesenak, M. β-Glucans: Multi-Functional Modulator of Wound Healing. Molecules 2018, 23, 806. [Google Scholar] [CrossRef] [Green Version]
- Engstad, R.E.; Robertsen, B. Specificity of a β-glucan receptor on macrophages from Atlantic salmon (Salmo salar L.). Dev. Comp. Immunol. 1994, 18, 397–408. [Google Scholar] [CrossRef]
- Engstad, C.S.; Engstad, R.E.; Olsen, J.; Østerud, B. The effect of soluble β-1,3-glucan and lipopolysaccharide on cytokine production and coagulation activation in whole blood. Int. Immunopharmacol. 2002, 2, 1585–1597. [Google Scholar] [CrossRef]
- Zykova, S.N.; Balandina, K.A.; Vorokhobina, N.V.; Kuznetsova, A.V.; Engstad, R.; Zykova, T.A. Macrophage stimulating agent soluble yeast β-1,3/1,6-glucan as a topical treatment of diabetic foot and leg ulcers: A randomized, double blind, placebo-controlled phase II study. J. Diabetes Investig. 2014, 5, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Gianino, E.; Miller, C.; Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering 2018, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurler, J.; Berg, O.A.; Skar, M.; Conradi, A.H.; Johnsen, P.J.; Škalko-Basnet, N. Improved Burns Therapy: Liposomes-in-Hydrogel Delivery System for Mupirocin. J. Pharm. Sci. 2012, 101, 3906–3915. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, L.M.; Giordani, B.; Pettersen, A.K.; Vitali, B.; Basnet, P.; Škalko-Basnet, N. Liposomes-in-chitosan hydrogel boosts potential of chlorhexidine in biofilm eradication in vitro. Carbohydr. Polym. 2021, 262, 117939. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Taxipalati, M.; Zhang, A.; Que, F.; Wei, H.; Feng, F.; Zhang, H. Electrospun Chitosan/Poly(ethylene oxide)/Lauric Arginate Nanofibrous Film with Enhanced Antimicrobial Activity. J. Agric. Food Chem. 2018, 66, 6219–6226. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, S.; Zare, M.R.; Shojaei, F.; Zareshahrabadi, Z.; Koohi-Hosseinabadi, O.; Saharkhiz, M.J.; Iraji, A.; Zomorodian, K.; Khorram, M. Core-shell chitosan/PVA-based nanofibrous scaffolds loaded with Satureja mutica or Oliveria decumbens essential oils as enhanced antimicrobial wound dressing. Int. J. Pharm. 2021, 597, 120288. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Gimbun, J.; Ramakrishnan, P.; Ranganathan, B.; Reddy, S.M.M.; Shanmugam, G. Effect of Solution Properties and Operating Parameters on Needleless Electrospinning of Poly (Ethylene Oxide) Nanofibers Loaded with Bovine Serum Albumin. Curr. Drug Deliv. 2019, 16, 913–922. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. A fundamental study of chitosan/PEO electrospinning. Polymer 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J.; Mašek, J. Hypromellose–A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Stie, M.B.; Jones, M.; Sorensen, H.O.; Jacobsen, J.; Chronakis, I.S.; Nielsen, H.M. Acids ’generally recognized as safe’ affect morphology and biocompatibility of electrospun chitosan/polyethylene oxide nanofibers. Carbohydr. Polym. 2019, 215, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, D.L.; Hammouda, B.; Kline, S.R.; Chen, W.-R. Unusual Phase Behavior in Mixtures of Poly(ethylene oxide) and Ethyl Alcohol. J. Polym. Sci. B Polym. Phys. 2006, 44, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Rasband, W.S. ImageJ; National Institutes of Health: Bethesda, MD, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 19 July 2021).
- ASTM D882-18. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar] [CrossRef]
- Bradford, C.; Freeman, R.; Percival, S.L. In Vitro Study of Sustained Antimicrobial Activity of a New Silver Alginate Dressing. J. Am. Col. Certif. Wound. Spec. 2009, 1, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Joraholmen, M.W.; Vanic, Z.; Tho, I.; Škalko-Basnet, N. Chitosan-coated liposomes for topical vaginal therapy: Assuring localized drug effect. Int. J. Pharm. 2014, 472, 94–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Amiri Darban, S.; Fazly Bazzaz, B.S.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656. [Google Scholar] [CrossRef]
- Cauzzo, J.; Nystad, M.; Holsæter, A.M.; Basnet, P.; Škalko-Basnet, N. Following the Fate of Dye-Containing Liposomes In Vitro. Int. J. Mol. Sci. 2020, 21, 4847. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Akinalan Balik, B.; Argin, S. Role of rheology on the formation of Nanofibers from pectin and polyethylene oxide blends. J. Appl. Polym. Sci. 2019, 137, 48294. [Google Scholar] [CrossRef]
- Mirtič, J.; Balažic, H.; Zupančič, Š.; Kristl, J. Effect of Solution Composition Variables on Electrospun Alginate Nanofibers: Response Surface Analysis. Polymers 2019, 11, 692. [Google Scholar] [CrossRef] [Green Version]
- Rošic, R.; Pelipenko, J.; Kocbek, P.; Baumgartner, S.; Bešter-Rogač, M.; Kristl, J. The role of rheology of polymer solutions in predicting nanofiber formation by electrospinning. Eur. Polym. J. 2012, 48, 1374–1384. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Klossner, R.R.; Queen, H.A.; Coughlin, A.J.; Krause, W.E. Correlation of Chitosan’s Rheological Properties and Its Ability to Electrospin. Biomacromolecules 2008, 9, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Abdelgawad, A.M.; Salas, C.; Rojas, O.J. Curdlan in fibers as carriers of tetracycline hydrochloride: Controlled release and antibacterial activity. Carbohydr. Polym. 2016, 154, 194–203. [Google Scholar] [CrossRef]
- Ni Annaidh, A.; Bruyere, K.; Destrade, M.; Gilchrist, M.D.; Ottenio, M. Characterization of the anisotropic mechanical properties of excised human skin. J. Mech. Behav. Biomed. Mater. 2012, 5, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junker, J.P.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Ousey, K.; Cutting, K.; Rogers, A.A.; Rippon, M.G. The Importance of Hydration in Wound Healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Stie, M.B.; Gatke, J.R.; Wan, F.; Chronakis, I.S.; Jacobsen, J.; Nielsen, H.M. Swelling of mucoadhesive electrospun chitosan/polyethylene oxide nanofibers facilitates adhesion to the sublingual mucosa. Carbohydr. Polym. 2020, 242, 116428. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Peh, K.K.; Ch’ng, H.S. Mechanical, Bioadhesive Strength and Biological Evaluations of Chitosan films for Wound Dressing. J. Pharm. Sci. 2000, 3, 303–311. [Google Scholar]
- Iacob, A.-T.; Drăgan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An Overview of Biopolymeric Electrospun Nanofibers Based on Polysaccharides for Wound Healing Management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef]
- Altun, E.; Yuca, E.; Ekren, N.; Kalaskar, D.M.; Ficai, D.; Dolete, G.; Ficai, A.; Gunduz, O. Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery. Pharmaceutics 2021, 13, 613. [Google Scholar] [CrossRef]
- Graça, M.F.P.; de Melo-Diogo, D.; Correia, I.J.; Moreira, A.F. Electrospun Asymmetric Membranes as Promising Wound Dressings: A Review. Pharmaceutics 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shi, Z.; Wan, X.; Fang, H.; Yu, D.G.; Chen, X.; Liu, P. The Relationships between Process Parameters and Polymeric Nanofibers Fabricated Using a Modified Coaxial Electrospinning. Nanomaterials 2019, 9, 843. [Google Scholar] [CrossRef] [Green Version]
- Piipponen, M.; Li, D.; Landen, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- ISO 10993-5:2009. Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef]
- Abid, S.; Hussain, T.; Nazir, A.; Zahir, A.; Ramakrishna, S.; Hameed, M.; Khenoussi, N. Enhanced antibacterial activity of PEO-chitosan nanofibers with potential application in burn infection management. Int. J. Biol. Macromol. 2019, 135, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tondervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, L.J.R.; Butt, J. Chitosan films are NOT antimicrobial. Biotechnol. Lett. 2011, 33, 417–421. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, B.; Lin, C.; Bian, Z.; Xu, B. An insight into anti-inflammatory effects of fungal beta-glucans. Trends Food Sci. Technol. 2015, 41, 49–59. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, Y.Y.; Wu, G.J.; Huang, C.H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Rawlingson, A. Nitric oxide, inflammation and acute burn injury. Burns 2003, 29, 631–640. [Google Scholar] [CrossRef]





| Polymer Solutions | Ingredients | ||||
|---|---|---|---|---|---|
| βG (%) | CHI (%) | PEO (%) | HPMC (%) | CAM (%) | |
| βG-CHI-sol | 20.0 | 20.0 | 30.0 | 30.0 | - |
| βG-sol | 20.0 | - | 40.0 | 40.0 | - |
| CHI-sol | - | 20.0 | 40.0 | 40.0 | - |
| Copol-sol | - | - | 50.0 | 50.0 | - |
| βG-CHI-CAM-sol | 20.0 | 20.0 | 29.5 | 29.5 | 1.0 |
| βG-CAM-sol | 20.0 | - | 39.5 | 39.5 | 1.0 |
| CHI-CAM-sol | - | 20.0 | 39.5 | 39.5 | 1.0 |
| Copol-CAM-sol | - | - | 49.5 | 49.5 | 1.0 |
| Polymer Solutions | Solution Characteristics | |||
|---|---|---|---|---|
| pH | Surface Tension (mN/m) | Conductivity (µS/cm) | Viscosity (Pa·s) | |
| βG-CHI-sol | 4.62 ± 0.03 | 27.4 ± 0.5 | 143.2 ± 18.2 | 2.37 ± 0.52 |
| βG-sol | 3.89 ± 0.01 | 26.6 ± 0.4 | 64.5 ± 1.6 | 1.23 ± 0.15 |
| CHI-sol | 4.57 ± 0.01 | 26.9 ± 0.5 | 138.4 ± 3.3 | 2.30 ± 0.02 |
| Copol-sol | 3.91 ± 0.09 | 26.4 ± 0.4 | 71.0 ± 1.2 | 1.09 ± 0.08 |
| βG-CHI-CAM-sol | 4.53 ± 0.03 | 27.9 ± 0.4 | 143.3 ± 11.5 | 2.55 ± 0.28 |
| βG-CAM-sol | 3.78 ± 0.06 | 26.8 ± 0.8 | 65.1 ± 2.0 | 1.17 ± 0.15 |
| CHI-CAM-sol | 4.52 ± 0.03 | 27.6 ± 1.0 | 148.9 ± 7.5 | 2.34 ± 0.25 |
| Copol-CAM-sol | 3.92 ± 0.11 | 27.1 ± 1.0 | 70.1 ± 1.6 | 1.09 ± 0.20 |
| Formulation | Nanofiber Characteristics and Mechanical Properties | ||
|---|---|---|---|
| Thickness (µm) | Tensile Strength (MPa) | Elongation at Break (%) | |
| βG-CHI-nf | 35.1 ± 8.7 | 21.4 ± 18.7 | 3.5 ± 0.8 |
| βG-nf | 71.7 ± 11.8 | 21.8 ± 13.6 | 4.8 ± 1.4 |
| CHI-nf | 47.3 ± 5.1 | 17.1 ± 3.7 | 7.3 ± 0.9 |
| Copol-nf | 64.3 ± 7.9 | 21.2 ± 12.0 | 8.5 ± 0.6 |
| βG-CHI-CAM-nf | 38.0 ± 1.2 | 12.2 ± 7.6 | 5.4 ± 2.1 |
| βG-CAM-nf | 85.8 ± 11.2 | 9.2 ± 4.2 | 3.9 ± 0.7 |
| CHI-CAM-nf | 55.5 ± 7.2 | 20.8 ± 6.2 | 8.9 ± 1.8 |
| Cop-CAM-nf | 72.7 ± 9.0 | 15.7 ± 4.3 | 6.7 ± 0.9 |
| Formulation | Swelling Index (%) |
|---|---|
| βG-CHI-CAM-nf | 1055 ± 318 |
| βG-CAM-nf * | - |
| CHI-CAM-nf | 779 ± 242 |
| Cop-CAM-nf * | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulte-Werning, L.V.; Murugaiah, A.; Singh, B.; Johannessen, M.; Engstad, R.E.; Škalko-Basnet, N.; Holsæter, A.M. Multifunctional Nanofibrous Dressing with Antimicrobial and Anti-Inflammatory Properties Prepared by Needle-Free Electrospinning. Pharmaceutics 2021, 13, 1527. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13091527
Schulte-Werning LV, Murugaiah A, Singh B, Johannessen M, Engstad RE, Škalko-Basnet N, Holsæter AM. Multifunctional Nanofibrous Dressing with Antimicrobial and Anti-Inflammatory Properties Prepared by Needle-Free Electrospinning. Pharmaceutics. 2021; 13(9):1527. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13091527
Chicago/Turabian StyleSchulte-Werning, Laura Victoria, Anjanah Murugaiah, Bhupender Singh, Mona Johannessen, Rolf Einar Engstad, Nataša Škalko-Basnet, and Ann Mari Holsæter. 2021. "Multifunctional Nanofibrous Dressing with Antimicrobial and Anti-Inflammatory Properties Prepared by Needle-Free Electrospinning" Pharmaceutics 13, no. 9: 1527. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics13091527