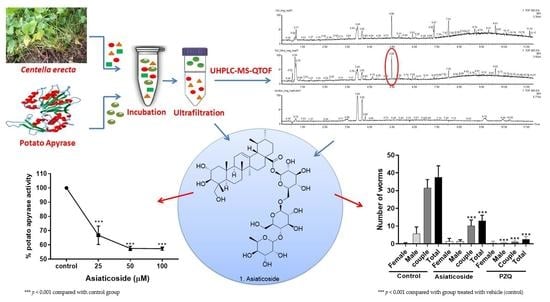
Identification of Asiaticoside from Centella erecta (Apiaceae) as Potential Apyrase Inhibitor by UF-UHPLC-MS and Its In Vivo Antischistosomal Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Extraction and Preparation
2.3. Optimal Incubation Conditions with Cardamonin and Potato Apyrase by HPLC-DAD
2.4. Screening of Apyrase Inhibitors by Ultrafiltration and UHPLC-QTOF-MS-Based Binding Assay
2.5. Annotation of Potential Apyrase Ligands by Ultrafiltration Coupled to UHPLC-ESI-QTOF-MS
2.6. Isolation of Asiaticoside from C. erecta Extract
2.7. Potato Apyrase Inhibitory Assay
2.8. In Silico Analysis and Molecular Docking of Potato Apyrase, SmNTPDases 1 and 2
2.8.1. Three-Dimensional Structure of Target Proteins
2.8.2. Molecular Docking Simulations
2.8.3. Calculated Inhibition Constant
2.9. In Vivo Antischistosomal Studies
2.9.1. Animals and Parasite Maintenance
2.9.2. In Vivo Antischistosomal Studies
2.9.3. Randomization and Blinding
2.9.4. Statistical Analysis
3. Results
3.1. Optimization of Incubation Conditions with Potato Apyrase
3.2. Identification of Potato Apyrase Ligands Using Ultrafiltration UHPLC-MS-QTOF Analysis
3.3. Isolation of Asiaticoside from C. erecta Extract
3.4. Determination of Apyrase Activity of Asiaticoside
3.5. Molecular Docking Analysis of Asiaticoside against Potato Apyrase, SmNTPDase 1 and SmNTPDase 2
3.6. In Vivo Antischistosomal Studies of Asiaticoside against S. mansoni in Patent Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lago, E.M.; Xavier, R.P.; Teixeira, T.R.; Silva, L.M.; Da Silva Filho, A.A.; De Moraes, J. Antischistosomal agents: State of art and perspectives. Future Med. Chem. 2018, 10, 89–120. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.S.; Mengarda, A.C.; Miguel, F.B.; Enes, K.B.; Rodrigues, V.C.; Espírito-Santo, M.C.C.; Siyadatpanah, A.; Wilairatana, P.; Couri, M.R.C.; Moraes, J. Pyrazoline derivatives as promising novel antischistosomal agents. Sci. Rep. 2021, 11, 23437. [Google Scholar] [CrossRef]
- Porto, R.; Mengarda, A.C.; Cajas, R.A.; Salvadori, M.C.; Teixeira, F.S.; Arcanjo, D.D.R.; Siyadatpanah, A.; Pereira, M.L.; Wilairatana, P.; Moraes, J. Antiparasitic Properties of Cardiovascular Agents against Human Intravascular Parasite Schistosoma mansoni. Pharmaceuticals 2021, 14, 686. [Google Scholar] [CrossRef] [PubMed]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.; Gartner, F.; Costa, J.M.C. Praziquantel for Schistosomiasis: Single-Drug Metabolism Revisited, Mode of Action, and Resistance. Antimicrob. Agents Chemother. 2017, 61, e02582-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehne, E.; Zander, N.; Rodi, M.; Held, J.; Hoffmann, W.; Zoleko-Manego, R.; Ramharter, M.; Mombo-Ngoma, G.; Kremsner, P.G.; Kreidenweiss, A. Evidence for in vitro and in vivo activity of the antimalarial pyronaridine against Schistosoma. PLoS Negl. Trop. Dis. 2021, 15, e0009511. [Google Scholar] [CrossRef]
- Silva, L.M.; Marconato, D.G.; da Silva, M.P.N.; Raposo, N.R.B.; Silva-Facchini, G.F.; Macedo, G.C.; Teixeira, F.S.; Salvadori, M.C.B.S.; Faria-Pinto, P.; Moraes, J.; et al. Licochalcone A-loaded solid lipid nanoparticles improve antischistosomal activity in vitro and in vivo. Nanomedicine 2021, 19, 1641–1655. [Google Scholar] [CrossRef]
- De Carvalho, L.S.A.; Junior, I.J.A.; Junqueira, L.R.; Silva, L.M.; Riani, L.R.; Faria-Pinto, P.; Da Silva Filho, A.A. ATP-Diphosphohydrolases in parasites: Localization, functions and recent developments in drug Discovery. Curr. Protein Pept. Sci. 2019, 20, 873–884. [Google Scholar] [CrossRef]
- De Carvalho, L.S.A.; Geraldo, R.B.; De Moraes, J.; Silva Pinto, P.L.; De Faria-Pinto, P.; Pereira, O.S.; Da Silva Filho, A.A. Schistosomicidal activity and docking of Schistosoma mansoni ATPDase 1 with licoflavone B isolated from Glycyrrhiza inflata (Fabaceae). Exp. Parasitol. 2015, 159, 207–214. [Google Scholar] [CrossRef]
- Faria-Pinto, P.; Rezende-Soares, F.A.; Molica, A.M.; Montesano, M.A.; Marques, M.J.; Rocha, M.O.; Gomes, J.A.; Enk, M.J.; Correa-Oliveira, R.; Coelho, P.M.; et al. Mapping of the conserved antigenic domains shared between potato apyrase and parasite ATP diphosphohydrolases: Potential application in human parasitic diseases. Parasitology 2008, 135, 943–953. [Google Scholar] [CrossRef]
- Vasconcelos, E.G.; Ferreira, S.T.; De Carvalho, T.M.U.; De Souza, W.; Kettlun, A.M.; Mancilla, M.; Valenzuela, M.A.; Verjovski-Almeida, S. Partial purification and immunohistochemical localization of ATP Diphosphohydrolase from Schistosoma mansoni: Immunological cross-reactivities with potato apyrase and Toxoplasma gondii nucleoside triphosphate hydrolase. J. Biol. Chem. 1996, 271, 22139–22145. [Google Scholar] [CrossRef] [Green Version]
- Gusmão, M.A.N.; Júnior, S.M.; Marconato, D.G.; Emídio, N.B.; Farani, P.S.G.; Golner, A.M.; Araújo, N.; Coelho, P.M.Z.; Macedo, G.C.; Da Silva Filho, A.A.; et al. Potato apyrase reduces granulomatous area and increases presence of multinucleated giant cells in murine schistosomiasis. Parasitol. Int. 2021, 83, 102317. [Google Scholar] [CrossRef]
- De Faria Pinto, P.; Meirelle, M.N.L.; Lenzi, H.L.; Mota, E.M.; Penido, M.L.O.; Coelho, P.M.Z.; Vasconcelos, E.G. ATP Diphosphohydrolase from Schistosoma mansoni egg: Characterization and immunocytochemical localization of a new antigen. Parasitology 2004, 129, 51–57. [Google Scholar] [CrossRef]
- De Carvalho, L.S.A.; Silva, L.M.; De Souza, V.C.; Da Silva, M.P.N.; Capriles, P.V.S.Z.; Faria-Pinto, P.; De Moraes, J.; Da Silva Filho, A.A. Cardamonin presents in vivo activity against Schistosoma mansoni and inhibits potato apyrase. Chem. Biodivers. 2021, 18, e2100604. [Google Scholar] [CrossRef]
- Pereira, V.R.D.; Junior, I.J.A.; Da Silveira, L.S.; Geraldo, R.B.; De Faria Pinto, P.; Teixeira, F.S.; Salvadori, M.C.; Silva, M.P.; Alves, L.A.; Capriles, P.V.S.Z.; et al. In vitro and in vivo antischistosomal activities of chalcones. Chem. Biodivers. 2018, 15, e1800398. [Google Scholar] [PubMed]
- Farani, P.S.G.; Marconato, D.G.; Emídio, N.B.; Pereira, V.R.D.; Junior, I.J.A.; da Silveira, L.S.; Couri, M.R.C.; Vasconcelos, E.G.; Castro-Borges, W.; Da Silva Filho, A.A.; et al. Screening of plant derived chalcones on the inhibition of potato apyrase: Potential protein biotechnological applications in health. Int. J. Biol. Macromol. 2020, 164, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Rumalla, C.S.; Ali, Z.; Weerasooriya, A.D.; Smillie, T.J.; Khan, I.A. A new triterpene glycoside from Centella erecta. Fitoterapia 2010, 81, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Li, L.; Li, B.; Zheng, M.; Tsao, R. Ultrafiltration LC-ESI-MSn screening of 5-lipoxygenase inhibitors from selected Chinese medicinal herbs Saposhnikovia divaricata, Smilax glabra, Pueraria lobata and Carthamus tinctorius. J. Funct. Foods 2016, 24, 244–253. [Google Scholar] [CrossRef]
- Guo, Y.; Fu, R.; Qian, Y.; Zhou, Z.; Liu, H.; Qi, J.; Zhangc, B.; Yu, B. Comprehensive screening and identification of natural inducible nitric oxide synthase inhibitors from Radix Ophiopogonis by off-line multi-hyphenated analyses. J. Chromatogr. A 2019, 1592, 55–63. [Google Scholar] [CrossRef]
- Li, L.; Kong, J.; Yao, C.; Liu, X.; Liu, J. Rapid identification of urokinase plasminogen activator inhibitors from Traditional Chinese Medicines based on ultrafiltration, LC–MS and in silico docking. J. Pharm. Biomed. 2019, 164, 241–248. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Sun, L.; Wang, Y.; Gao, X.; Cheng, Y. An ultrafiltration high-performance liquid chromatography coupled with diode array detector and mass spectrometry approach for screening and characterizing tyrosinase inhibitors from mulberry leaves. Anal. Chim. Acta 2012, 719, 87–95. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, Z.; Chen, S.; Wang, L.; Xian, S. Rapid Screening of Potential Phosphodiesterase Inhibitors from the Roots of Ilex pubescens Hook. et Arn. Using a Combination of Ultrafiltration and LC–MS. Evid.-Based Complementary Altern. Med. 2017, 2017, 2749643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taussky, H.H.; Shorr, E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [CrossRef]
- De Souza, V.C.; Nunes, V.S.; Vasconcelos, E.G.; Faria-Pinto, P.; Capriles, P.V. Structural comparative analysis of secreted NTPDase models of Schistosoma mansoni and Homo sapiens. In Brazilian Symposium on Bioinformatics; Springer: Cham, Switzerland, 2014; pp. 91–98. [Google Scholar]
- Nunes, V.S.; Vasconcelos, E.G.; Faria-Pinto, P.; Borges, C.C.H.; Capriles, P.V. Structural comparative analysis of Ecto-NTPDase models from S. mansoni and H. sapiens. Lect. Notes Comput. Sci. 2015, 9096, 247–259. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Cryst. 2002, 40, 82–92. [Google Scholar]
- Phosrithong, N.; Ungwitayatorn, J. Molecular docking study on anticancer activity of plant-derived natural products. Med. Chem. Res. 2010, 19, 817–835. [Google Scholar] [CrossRef]
- De Moraes, J.; De Oliveira, R.N.; Costa, J.P.; Junior, A.L.; De Sousa, D.P.; Freitas, R.M.; Allegreti, S.M.; Pinto, P.L. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLoS Negl. Trop. Dis. 2014, 8, e2617. [Google Scholar] [CrossRef] [Green Version]
- Pereira, V.R.D.; da Silveira, L.S.; Mengarda, A.C.; Júnior, I.J.A.; da Silva, O.O.Z.; Miguel, F.B.; Silva, M.P.; Almeida, A.C.; Torres, D.S.; Faria Pinto, P.; et al. Antischistosomal properties of aurone derivatives against juvenile and adult worms of Schistosoma mansoni. Acta Trop. 2021, 213, 105741. [Google Scholar] [CrossRef]
- Guimarães, M.A.; De Oliveira, R.N.; De Almeida, R.L.; Mafud, A.C.; Sarkis, A.L.V.; Ganassin, R.; Da Silva, M.P.; Roquini, D.B.; Veras, L.M.; Sawada, T.C.H.; et al. Epiisopilosine alkaloid has activity against Schistosoma mansoni in mice without acute toxicity. PLoS ONE 2018, 13, e0196667. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Mendonça, P.S.; Morais, C.S.; Cogo, R.M.; Mazloum, S.F.; Salvadori, M.C.; Teixeira, F.S.; Morais, T.R.; Antar, G.M.; Lago, J.H.G.; et al. Antiparasitic activity of piplartine (piperlongumine) in a mouse model of schistosomiasis. Acta Trop. 2020, 205, 105350. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; de Oliveira, R.N.; Mengarda, A.C.; Roquini, D.B.; Allegretti, S.M.; Salvadori, M.C.; Teixeira, F.S.; De Sousa, D.P.; Pinto, P.L.S.; Da Silva Filho, A.A.; et al. Antiparasitic activity of nerolidol in a mouse model of schistosomiasis. Int. J. Antimicrob. Agents 2017, 50, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Xia, Y.; Dai, Y. Development and validation of high-performance liquid chromatography/electrospray ionization mass spectrometry for assay of madecassoside in rat plasma and its application to pharmacokinetic study. Biomed. Chromatogr. 2012, 26, 26–32. [Google Scholar] [CrossRef]
- Wang, T.; Leng, D.; Gao, F.; Jiang, C.; Xia, Y.; Dai, Y. A LC-ESI-MS method for the simultaneous determination of madecassoside and its metabolite madecassic acid in rat plasma: Comparison pharmacokinetics in normal and collagen-induced arthritic rats. Chin. J. Nat. Med. 2014, 12, 943–951. [Google Scholar] [CrossRef]
- Long, H.S.; Stander, M.A.; Van Wyk, B.E. Notes on the occurrence and significance of triterpenoids (asiaticoside and related compounds) and caffeoylquinic acids in Centella species. S. Afr. J. Bot. 2012, 82, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Zacaria, F.; Ibrahim, W.N.W.; Ismail, I.S.; Ahmad, H.; Manshoor, N.; Ismail, N.; Zaina, Z.; Shaari, K. LCMS/MS Metabolite Profiling and Analysis of Acute Toxicity Effect of the Ethanolic Extract of Centella asiatica on Zebrafish Model. Pertanika J. Sci. Technol. 2019, 27, 971–989. [Google Scholar]
- Maulidiani, H.; Khatib, A.; Shaari, K.; Abas, F.; Shitan, M.; Kneer, R.; Neto, V.; Lajis, N.H. Discrimination of Three Pegaga (Centella) Varieties and Determination of Growth-Lighting Effects on Metabolites Content Based on the Chemometry of 1H Nuclear Magnetic Resonance Spectroscopy. J. Agric. Food Chem. 2012, 60, 410–417. [Google Scholar]
- Zebisch, M.; Sträter, N. Structural insight into signal conversion and inactivation by ntpdase2 in purinergic signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 6882–6887. [Google Scholar] [CrossRef] [Green Version]
- Kozakiewicz, A.; Neumann, P.; Banach, M.; Komoszyński, M.; Wojtczak, A. Modeling studies of potato nucleoside triphosphate diphosphohydrolase NTPDase1: An insight into the catalytic mechanism. Acta Biochim. Pol. 2008, 55, 141–150. [Google Scholar] [CrossRef]
- Filho, C.A.L.M.; Barbosa, M.O.; Oliveira, A.R.; Santiago, E.F.; de Souza, V.C.A.; Lucena, J.P.; Fernandes, C.J.B.; dos Santos, I.R.; Leão, R.L.C.; dos Santos, F.A.B.; et al. In vitro and in vivo activities of multi-target phtalimido-thiazoles on Schistosomiasis mansoni. Eur. J. Pharm. Sci. 2020, 146, 105236. [Google Scholar] [CrossRef]
- Huai, J.; Zhao, X.; Wang, S.; Xie, L.; Li, Y.; Zhang, T.; Cheng, C.; Dai, R. Characterization and screening of cyclooxygenase-2 inhibitors from Zi-shen pill by affinity ultrafiltration-ultra performance liquid chromatography mass spectrometry. J. Ethnopharmacol. 2019, 241, 111900. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Z.; Quispe, Y.N.G.; Hwang, S.H.; Kim, H.Y.; Kang, B.G.; Lim, S.S. Target guided isolation of potential tyrosinase inhibitors from Otholobium pubescens (Poir.) J.W. Grimes by ultrafiltration, high-speed countercurrent chromatography and preparative HPLC. Ind. Crops Prod. 2019, 134, 195–205. [Google Scholar] [CrossRef]
- Torbati, F.A.; Ramezani, M.; Dehghan, R.; Amiri, M.S.; Moghadam, A.T.; Shakour, N.; Elyasi, S.; Sahebkar, A.; Emami, S.A. Ethnobotany, phytochemistry and pharmacological features of Centella asiatica: A comprehensive review. In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health, 1st ed.; Barreto, G.E., Sahebkar, A., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1308, pp. 451–499. [Google Scholar]
- Prasesti, G.K.; Kurniati, N.F. Toxicity studies of Centella asiatica for drug development: Mini review. Biointerface Res. Appl. Chem. 2022, 12, 8081–8093. [Google Scholar]
- Deshpande, P.O.; Mohan, V.; Thakurdesai, P. Preclinical safety assessment of standardized extract of Centella asiatica (L.) urban leaves. Toxicol. Int. 2015, 22, 10–20. [Google Scholar] [PubMed] [Green Version]
- Vo, N.N.Q.; Fukushima, E.O.; Muranaka, T. Structure and hemolytic activity relationships of triterpenoid saponins and sapogenins. J. Nat. Med. 2017, 71, 50–58. [Google Scholar] [CrossRef]
- Melek, F.R.; Tadros, M.M.; Yousif, F.; Selim, M.A.; Hassan, M.H. Screening of marine extracts for schistosomicidal activity in vitro. Isolation of the triterpene glycosides echinosides A and B with potential activity from the Sea Cucumbers Actinopyga echinites and Holothuria polii. Pharm. Biol. 2012, 50, 490–496. [Google Scholar] [CrossRef]
- Jisaka, M.; Kawanaka, M.; Sugiyama, H.; Takegawa, K.; Huffman, M.A.; Ohigashi, H.; Koshimizu, Z. Antischistosomal activities of sesquiterpene lactones and steroid glucosides from Vernonia amygdalina, possibly used by wild chimpanzees against parasite-related diseases. Biosci. Biotechnol. Biochem. 1992, 56, 845–846. [Google Scholar] [CrossRef]
- Kang, N.; Shen, W.; Gao, H.; Feng, Y.; Zhu, W.; Yang, S.; Liu, Y.; Xu, Q.; Yu, D. Antischistosomal Properties of Hederacolchiside A1 Isolated from Pulsatilla chinensis. Molecules 2018, 23, 1431. [Google Scholar] [CrossRef] [Green Version]
- Costain, A.H.; MacDonald, A.S.; Smits, H.H. Schistosome Egg Migration: Mechanisms, Pathogenesis and Host Immune Responses. Front. Immunol. 2018, 9, 3042. [Google Scholar] [CrossRef] [Green Version]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Da’dara, A.A.; Bhardwaj, R.; Skelly, P.J. Schistosome apyrase SmATPDase1, but not SmATPDase2, hydrolyses exogenous ATP and ADP. Purinergic Signal. 2014, 10, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic potential of Centella asiatica and its triterpenes: A review. Front. Pharmacol. 2020, 11, 1373. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Bollen, M.; David, J.; Speers, A.B.; Brandes, M.S.; Gray, N.E.; Magaña, A.A.; McClure, C.; Stevens, J.F.; Maier, C.S.; et al. Pharmacokinetics and pharmacodynamics of key components of a standardized Centella asisatica product in cognitively impaired older adults: A phase 1, double-blind, randomized clinical trial. Antioxidants 2022, 11, 215. [Google Scholar] [CrossRef] [PubMed]







| Peak | Proposed Compounds | Rt (min) | m/z Experimental [M − H]− | Main Fragments via MS/MS | Molecular Formula | References |
|---|---|---|---|---|---|---|
| 1 | Asiaticoside | 4.98 | 993.4827 | 957.5045; 487.3400; 469.1537; 162.8393; 160.8240; 116.9285 | C48H78O19 | [36,37] |
| 2 | Madecassic acid | 6.87 | 503.3387 | 325.1851; 178.8417; 162.8393; 160.8420; 116.9285 | C30H48O6 | [36,37] |
| 3 | Asiatic acid | 7.52 | 487.3445 | 441.2468; 325.1851; 178.8417; 162.8419; 160.8420; 116.9285 | C30H48O5 | [36,37] |
| Protein | Energy a | cKib | Interactions |
|---|---|---|---|
| Apyrase | −9.9 | 0.055 | T30, E145, D172, S312, Q179, W408 |
| SmNTPDase 1 | −10.0 | 0.047 | S81, T154, E201, D232, K279, W483 |
| SmNTPDase 2 | −11.3 | 0.005 | T48, R51, H53, H76, E164, D203, E466, N468, W469 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Carvalho, L.S.A.; de Souza, V.C.; Rodrigues, V.C.; Ribeiro, A.C.; Nascimento, J.W.L.; Capriles, P.V.S.Z.; Pinto, P.d.F.; de Moraes, J.; da Silva Filho, A.A. Identification of Asiaticoside from Centella erecta (Apiaceae) as Potential Apyrase Inhibitor by UF-UHPLC-MS and Its In Vivo Antischistosomal Activity. Pharmaceutics 2022, 14, 1071. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051071
de Carvalho LSA, de Souza VC, Rodrigues VC, Ribeiro AC, Nascimento JWL, Capriles PVSZ, Pinto PdF, de Moraes J, da Silva Filho AA. Identification of Asiaticoside from Centella erecta (Apiaceae) as Potential Apyrase Inhibitor by UF-UHPLC-MS and Its In Vivo Antischistosomal Activity. Pharmaceutics. 2022; 14(5):1071. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051071
Chicago/Turabian Stylede Carvalho, Lara Soares Aleixo, Vinícius Carius de Souza, Vinícius C. Rodrigues, Aline Correa Ribeiro, Jorge Willian Leandro Nascimento, Priscila V. S. Z. Capriles, Priscila de F. Pinto, Josué de Moraes, and Ademar Alves da Silva Filho. 2022. "Identification of Asiaticoside from Centella erecta (Apiaceae) as Potential Apyrase Inhibitor by UF-UHPLC-MS and Its In Vivo Antischistosomal Activity" Pharmaceutics 14, no. 5: 1071. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051071