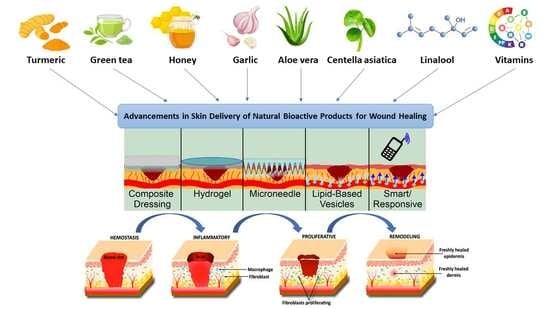
Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades
Abstract
:1. Introduction
2. Wound-Healing Physiology
3. Conventional Treatment
3.1. TIME and TWA: Deciding on a Treatment
3.2. Wound-Healing Dressings
4. Natural Products
4.1. Modulators of Cellular Activity
4.2. Modulators of Collagen Synthesis
4.3. Modulators of Angiogenesis
4.4. Modulators of the Extracellular Matrix
4.5. Modulators of Cytokines and Growth Factors
4.6. Natural Products Acting as Antibiotics or Antimicrobials
4.7. Modulators of the Oxidant–Antioxidant Balance of the Wound Microenvironment
4.8. Other Natural Products with Wound-Healing Properties
5. Advanced Delivery Strategies for Natural Wound-Healing Compounds
5.1. Composite Dressings
5.2. Hydrogels
5.3. Microneedles
5.4. Lipid-Based Vesicles/Nanotech
5.5. Responsive and Smart Delivery
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yi, X.; Yu, X.; Yuan, Z. A Novel Bacterial Biofilms Eradication Strategy Based on the Microneedles with Antibacterial Properties. Procedia CIRP 2020, 89, 159–163. [Google Scholar] [CrossRef]
- Barnum, L.; Samandari, M.; Schmidt, T.A.; Tamayol, A. Microneedle Arrays for the Treatment of Chronic Wounds. Exp. Opin. Drug Deliv. 2020, 17, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal Plants and their Natural Components as Future Drugs for the Treatment of Burn Wounds: An Integrative Review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Li, M.; Lu, Y.; Liu, D.; Li, C. Burn Wound Healing Properties of Asiaticoside and Madecassoside. Exp. Ther. Med. 2016, 12, 1269–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Assembly. Traditional Medicine; WHO: Geneva, Switzerland, 2014; Volume 9.1. [Google Scholar]
- Liu, S.; Chuang, W.; Lam, W.; Jiang, Z.; Cheng, Y. Safety Surveillance of Traditional Chinese Medicine: Current and Future. Drug Saf. 2015, 38, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Ahmad Khan, M.S.; Ahmad, I. Chapter 1—Herbal Medicine: Current Trends and Future Prospects. In New Look to Phytomedicine; Ahmad Khan, M.S., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 3–13. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Harwansh, R.K.; Bhattacharyya, S. Chapter 10—Bioavailability of Herbal Products: Approach toward Improved Pharmacokinetics. In Evidence-Based Validation of Herbal Medicine; Mukherjee, P.K., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 217–245. [Google Scholar]
- Lang, W.; Mennicke, W.H. Pharmacokinetic Studies on Triatiated Aescin in the Mouse and Rat. Arzneimittelforschung 1972, 22, 1928–1932. [Google Scholar]
- Carey, B. When Trust in Doctors Erodes, Other Treatments Fill the Void. The New York Times, 3 February 2006; p. A-1. [Google Scholar]
- Dai, C.; Shih, S.; Khachemoune, A. Skin Substitutes for Acute and Chronic Wound Healing: An Updated Review. J. Dermatol. Treat. 2020, 31, 639–648. [Google Scholar] [CrossRef]
- Verma, N.; Kumari, U.; Mittal, S.; Mittal, A.K. Effect of Asiaticoside on the Healing of Skin Wounds in the Carp Cirrhinus Mrigala: An Immunohistochemical Investigation. Tissue Cell 2017, 49, 734–745. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The Humanistic and Economic Burden of Chronic Wounds: A Systematic Review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, H.; Lee, M.H.; Yuo, K.E.; Kwon, B.; Seo, H.J.; Park, J. Asiaticoside Enhances Normal Human Skin Cell Migration, Attachment and Growth in Vitro Wound Healing Model. Phytomedicine 2012, 19, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhao, R.; Lin, H.; Jackson, C. Delivery Systems of Current Biologicals for the Treatment of Chronic Cutaneous Wounds and Severe Burns. Adv. Drug Deliv. Rev. 2018, 129, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and Innovative Approaches for Wound Healing and Skin Regeneration: Current Status and Advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef] [PubMed]
- Gray, K. TIME Wounds Will Health; Pharmac: Wellington, New Zealand, 2017. [Google Scholar]
- Cockbill, S.M.E.; Turner, T.D. The development of wound management products. In Chronic Wound Care: The Essentials E-Book; Krasner, D.L., van Rijswijk, L., Eds.; HMP: Melvern, KS, USA, 2018; pp. 145–164. [Google Scholar]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sun, Z.; Lee, J.; Kim, H.; Fu, X.; Leong, K.W. Advanced Drug Delivery Systems and Artificial Skin Grafts for Skin Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 209–239. [Google Scholar] [CrossRef]
- Romanelli, M.; Dowsett, C.; Doughty, D.; Senet, P.; Munter, C.; Martinez, J.L.L. Advances in Wound Care: The Triangle of Wound Assessment; World Union of Wound Healing Societies: London, UK, 2016. [Google Scholar]
- Clements, D. Skin and Wound Care Manual; St. Clare’s Mercy Hospital: St. John’s, NL, Canada, 2008. [Google Scholar]
- Hawkins, M. Volume D—Nursing Standards, Policies & Procedures. In Wound Care; Canterbury DHB: Christchurch, New Zealand, 2009; pp. 303–381. [Google Scholar]
- DermNet, N.Z. Wound Dressings; New Zealand Dermatological Society Incorporated: Wellington, New Zealand, 2009; Volume 2021. [Google Scholar]
- Cui, X.; Lee, J.J.L.; Chen, W.N. Eco-Friendly and Biodegradable Cellulose Hydrogels Produced from Low Cost Okara: Towards Non-Toxic Flexible Electronics. Sci. Rep. 2019, 9, 18166. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, H.; Yu, B.; Li, W.; Gao, F.; Zhang, K.; Zhang, H.; Shen, Y.; Cong, H. Chitosan Composite Hydrogels Cross-Linked by Multifunctional Diazo Resin as Antibacterial Dressings for Improved Wound Healing. J. Biomed. Mater. Res. A 2020, 108, 1890–1898. [Google Scholar] [CrossRef]
- Song, R.; Zheng, J.; Liu, Y.; Tan, Y.; Yang, Z.; Song, X.; Yang, S.; Fan, R.; Zhang, Y.; Wang, Y. A Natural Cordycepin/Chitosan Complex Hydrogel with Outstanding Self-Healable and Wound Healing Properties. Int. J. Biol. Macromol. 2019, 134, 91–99. [Google Scholar] [CrossRef]
- Yan, W.; Banerjee, P.; Liu, Y.; Mi, Z.; Bai, C.; Hu, H.; To, K.K.W.; Duong, H.T.T.; Leung, S.S.Y. Development of Thermosensitive Hydrogel Wound Dressing Containing Acinetobacter Baumannii Phage Against Wound Infections. Int. J. Pharm. 2021, 602, 120508. [Google Scholar] [CrossRef]
- Weller, C. Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 97–112. [Google Scholar] [CrossRef]
- Wietlisbach, C.M. 17—Wound Care. In Cooper’s Fundamentals of Hand Therapy, 3rd ed.; Wietlisbach, C.M., Ed.; Mosby: St. Louis, MO, USA, 2020; pp. 154–166. [Google Scholar]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of Polyurethane Foam Dressing Containing Silver and Asiaticoside for Healing of Dermal Wound. Asian J. Pharm. Sci. 2019, 14, 63–77. [Google Scholar] [CrossRef]
- He, M.; Ou, F.; Wu, Y.; Sun, X.; Chen, X.; Li, H.; Sun, D.; Zhang, L. Smart Multi-Layer PVA Foam/CMC Mesh Dressing with Integrated Multi-Functions for Wound Management and Infection Monitoring. Mater. Des. 2020, 194, 108913. [Google Scholar] [CrossRef]
- Koetse, M.; Rensing, P.; van Heck, G.; Sharpe, R.; Allard, B.; Wieringa, F.; Kruijt, P.; Meulendijks, N.; Jansen, H.; Schoo, H. In Plane Optical Sensor Based on Organic Electronic Devices. In Organic Field-Effect Transistors VII and Organic Semiconductors in Sensors and Bioelectronics; SPIE: Bellingham, WA, USA, 2008; p. 70541I. [Google Scholar] [CrossRef] [Green Version]
- Babikian, S.; Li, G.P.; Bachman, M. Integrated Bioflexible Electronic Device for Electrochemical Analysis of Blood. In Proceedings of the 2015 IEEE 65th Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 26–29 May 2015; pp. 685–690. [Google Scholar] [CrossRef]
- Duan, W.; Bian, X.; Bu, Y. Applications of Bioadhesives: A Mini Review. Front. Bioeng. Biotechnol. 2021, 9, 716035. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.; Cheng, B.; Mo, X.; Chen, C.; Pan, J. Moist-Retaining, Self-Recoverable, Bioadhesive, and Transparent in Situ Forming Hydrogels to Accelerate Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Dong, Z.; Tang, S.; Chu, W.; Zheng, X.; Zhen, L.; Chen, X.; Ding, C.; Luo, J.; Li, J. A Natural Polymer Based Bioadhesive with Self-Healing Behavior and Improved Antibacterial Properties. Biomater. Sci. 2020, 8, 4346–4357. [Google Scholar] [CrossRef]
- Barros Almeida, I.; Garcez Barretto Teixeira, L.; Oliveira de Carvalho, F.; Ramos Silva, É.; Santos Nunes, P.; Viana dos Santos Márcio, R.; de Souza Araújo, A.A. Smart Dressings for Wound Healing: A Review. Adv. Skin Wound Care 2021, 34, 1–8. [Google Scholar] [CrossRef]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal Wound Healing Processes with Curcumin Incorporated Collagen Films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- İnal, M.; Mülazımoğlu, G. Production and Characterization of Bactericidal Wound Dressing Material Based on Gelatin Nanofiber. Int. J. Biol. Macromol. 2019, 137, 392–404. [Google Scholar] [CrossRef]
- Bai, M.; Chen, M.; Yu, W.; Lin, J. Foam Dressing Incorporating Herbal Extract: An all-Natural Dressing for Potential use in Wound Healing. J. Bioact. Compat. Polym. 2017, 32, 293–308. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular Matrix Hydrogels from Decellularized Tissues: Structure and Function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin Improves Wound Healingby Modulating Collagen and Decreasing Reactive Oxygen Species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef]
- Panahi, Y.; Panahi, Y.; Khalili, N.; Khalili, N.; Sahebi, E.; Sahebi, E.; Namazi, S.; Namazi, S.; Karimian, M.; Karimian, M.; et al. Antioxidant Effects of Curcuminoids in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Inflammopharmacology 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, Q.; Zhang, H.; Cai, Q.; Li, X.; Shen, Z.; Zhu, W. A Shear-Thinning Electrostatic Hydrogel with Antibacterial Activity by Nanoengineering of Polyelectrolytes. Biomater. Sci. 2020, 8, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin Loaded Chitosan Polymer Based Nanoemulsion Gel: In Vitro, Ex Vivo Evaluation and in Vivo Wound Healing Studies. Int. J. Biol. Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From Ancient Medicine to Current Clinical Trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Frydman, G.H.; Olaleye, D.; Annamalai, D.; Layne, K.; Yang, I.; Kaafarani, H.M.A.; Fox, J.G. Manuka Honey Microneedles for Enhanced Wound Healing and the Prevention and/Or Treatment of Methicillin-Resistant Staphylococcus Aureus (MRSA) Surgical Site Infection. Sci. Rep. 2020, 10, 13229. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Honey, Wound Repair and Regenerative Medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Ranzato, E.; Martinotti, S.; Burlando, B. Honey Exposure Stimulates Wound Repair of Human Dermal Fibroblasts. Burn. Trauma 2013, 1, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Liu, X.; Wang, S.; Li, B.; Pan, T.; Liu, D.; Wang, F.; Diao, Y.; Li, K. Wound-Healing Promoting Effect of Total Tannins from Entada Phaseoloides (L.) Merr. in Rats. Burns 2017, 43, 830–838. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Dai, C.; Wang, Z.; Chen, W.; Liu, J.; Zhuo, R.; Yu, A.; Huang, S. A Novel Curcumin-Loaded Composite Dressing Facilitates Wound Healing due to its Natural Antioxidant Effect. Drug Des. Dev. Ther. 2019, 13, 3269–3280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, N.A.; Mohamed, M.; Mustafa, M.Z.; Zainuddin, A. In Vitro Modulation of Extracellular Matrix Genes by Stingless Bee Honey in Cellular Aging of Human Dermal Fibroblast Cells. J. Food Biochem. 2020, 44, e13098. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization during Wound Healing and its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, M.; Hunter, D.; Samman, S. Evaluation of the Nutritional and Metabolic Effects of Aloe vera. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; Chapter 3. [Google Scholar]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, M.P. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Lee, S.; Lee, M.; Lee, D.H.; Won, C.; Kim, S.M.; Chung, J.H. Dietary Aloe Vera Supplementation Improves Facial Wrinkles and Elasticity and it Increases the Type I Procollagen Gene Expression in Human Skin in Vivo. Ann. Dermatol. 2009, 21, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, M.D.; Beland, F.A. An Evaluation of the Biological and Toxicological Properties of Aloe Barbadensis (Miller), Aloe Vera. J. Environ. Sci. Health Part C 2006, 24, 103–154. [Google Scholar] [CrossRef] [Green Version]
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The Effect of Aloe Vera Clinical Trials on Prevention and Healing of Skin Wound: A Systematic Review. Iran. J. Med. Sci. 2019, 44, 1–9. [Google Scholar]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of Plant Derived Alkaloids and their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef] [Green Version]
- Fetse, J.P.; Kyekyeku, J.O.; Dueve, E.; Mensah, K.B. Wound Healing Activity of Total Alkaloidal Extract of the Root Bark of Alstonia Boonei (Apocynacea). Br. J. Pharm. Res. 2014, 4, 2642–2652. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. Front. Pharmacol. 2018, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Bonte, F.; Dumas, M.; Chaudagne, C.; Meybeck, A. Influence of Asiatic Acid, Madecassic Acid, and Asiaticoside on Human Collagen I Synthesis. Planta Med. 1994, 60, 133–135. [Google Scholar] [CrossRef]
- Maquart, F.; Bellon, G.; Gillery, P.; Wegrowski, Y.; Borel, J. Stimulation of Collagen Synthesis in Fibroblast Cultures by a Triterpene Extracted from Centella Asiatica. Connect. Tissue Res. 1990, 24, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological Review on Centella Asiatica: A Potential Herbal Cure-All. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.S.; Taher, M.; Mandal, U.K.; Jaffri, J.M.; Susanti, D.; Mahmood, S.; Zakaria, Z.A. Pharmacological Properties of Centella Asiatica Hydrogel in Accelerating Wound Healing in Rabbits. BMC Complementary Altern. Med. 2019, 19, 213. [Google Scholar] [CrossRef] [Green Version]
- Estrella-Mendoza, M.F.; Jiménez-Gómez, F.; López-Ornelas, A.; Pérez-Gutiérrez, R.M.; Flores-Estrada, J. Cucurbita Argyrosperma Seed Extracts Attenuate Angiogenesis in a Corneal Chemical Burn Model. Nutrients 2019, 11, 1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakiroglou, P.; VandenAkker, N.E.; Del Bo’, C.; Riso, P.; Klimis-Zacas, D. Role of Berry Anthocyanins and Phenolic Acids on Cell Migration and Angiogenesis: An Updated Overview. Nutrients 2019, 11, 1075. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Heilmann, J.; König, B. Natural Phenolic Metabolites with Anti-Angiogenic Properties—A Review from the Chemical Point of View. Beilstein J. Org. Chem. 2015, 11, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, B.; Malekzadeh, M.; Goodarzi, M.; Masoudifar, A.; Mirzaei, H. Green Tea and its Anti-Angiogenesis Effects. Biomed. Pharmacother. 2017, 89, 949–956. [Google Scholar] [CrossRef]
- Kusumo, W.D.; Mulyohadi, A.; Husnul, K.; Wibi, R.; Dianita, P.; Puspita, A.L.; Vanda, P. The Effect of Centella Asiatica to the Vascular Endothelial Growth Factor and Vascular Endothelial Growth Factor Receptor-2 on the Rotenone Induced Zebrafish Larvae (Danio Rerio) Stunting Model. GSC Biol. Pharm. Sci. 2018, 5, 88–95. [Google Scholar] [CrossRef]
- Majewska, I.; Gendaszewska-Darmach, E. Proangiogenic Activity of Plant Extracts in Accelerating Wound Healing—A New Face of Old Phytomedicines. Acta Biochim. Pol. 2011, 58, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.R.; Giacopelli, J.A. A Review of Wound Healing and Wound Dressing Products. J. Foot Ankle Surg. 1997, 36, 2–14. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Propolis: A New Frontier for Wound Healing? Burn. Trauma 2015, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The Role of the Extracellular Matrix Components in Cutaneous Wound Healing. BioMed Res. Int. 2014, 2014, 747584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olczyk, P.; Komosinska-Vassev, K.; Winsz-Szczotka, K.; Stojko, J.; Klimek, K.; Kozma, E.M. Propolis Induces Chondroitin/Dermatan Sulphate and Hyaluronic Acid Accumulation in the Skin of Burned Wound. Evid. Based Complementary Altern. Med. 2013, 2013, 290675. [Google Scholar] [CrossRef] [Green Version]
- Olczyk, P.; Komosińska-Vassev, K.; Winsz-Szczotka, K.; Koźma, E.M.; Wisowski, G.; Stojko, J.; Klimek, K.; Olczyk, K. Propolis Modulates Vitronectin, Laminin, and Heparan Sulfate/Heparin Expression during Experimental Burn Healing. J. Zhejiang Univ. Sci. B 2012, 13, 932–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, R.S.S.; Albuquerque-Júnior, R.L.C.; Araújo, A.A.S.; Almeida Jackson, R.G.S.; Santos, M.R.V.; Barreto, A.S.; DeSantana, J.M.; Siqueira-Lima, P.; Quintans, J.S.S.; Quintans-Júnior, L.J. A Systematic Review of the Wound-Healing Effects of Monoterpenes and Iridoid Derivatives. Molecules 2014, 19, 846–862. [Google Scholar] [CrossRef]
- Marques, F.M.; Figueira, M.M.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. In Vitro Anti-Inflammatory Activity of Terpenes via Suppression of Superoxide and Nitric Oxide Generation and the NF-κB Signalling Pathway. Inflammopharmacology 2019, 27, 281–289. [Google Scholar] [CrossRef]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril- Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound Healing Activity of A-Pinene and A-Phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef]
- Deardorff, R.; Spinale, F.G. Cytokines and Matrix Metalloproteinases as Potential Biomarkers in Chronic Heart Failure. Biomark. Med. 2009, 3, 513–523. [Google Scholar] [CrossRef] [Green Version]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Antioxidant Therapies for Wound Healing: A Clinical Guide to Currently Commercially Available Products. Ski. Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Rasco, B.A.; Jabal, J.M.F.; Aston, D.E.; Lin, M.; Konkel, M.E. Investigating Antibacterial Effects of Garlic (Allium Sativum) Concentrate and Garlic-Derived Organosulfur Compounds on Campylobacter Jejuni by using Fourier Transform Infrared Spectroscopy, Raman Spectroscopy, and Electron Microscopy. Appl. Environ. Microbiol. 2011, 77, 5257–5269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.; Lee, H.; Yoon, D.; Ji, D.; Kim, J.; Lee, C. Antioxidant and Antimicrobial Activities of Fresh Garlic and Aged Garlic by-Products Extracted with Different Solvents. Food Sci. Biotechnol. 2018, 27, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, B.J.; Tallian, K. Essential Oil of Lavender in Anxiety Disorders: Ready for Prime Time? Ment. Health Clin. 2017, 7, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Shewanella Putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of Antioxidant Activity of Vanillin by using Multiple Antioxidant Assays. Biochim. Biophys. Acta 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Arya, S.; Rookes, J.; Cahill, D.; Lenka, S. Vanillin: A Review on the Therapeutic Prospects of a Popular Flavouring Molecule. Adv. Trad Med. 2021, 21, 1. [Google Scholar] [CrossRef]
- Zhou, G.; Ruhan, A.; Ge, H.; Wang, L.; Liu, M.; Wang, B.; Su, H.; Yan, M.; Xi, Y.; Fan, Y. Research on a Novel Poly (Vinyl Alcohol)/Lysine/Vanillin Wound Dressing: Biocompatibility, Bioactivity and Antimicrobial Activity. Burns 2014, 40, 1668–1678. [Google Scholar] [CrossRef]
- Abdelmalek, M.; Spencer, J. Retinoids and Wound Healing. Dermatol. Surg. 2006, 32, 1219–1230. [Google Scholar] [CrossRef]
- Raber-Durlacher, J.; von Bültzingslöwen, I.; Logan, R.M.; Bowen, J.; Al-Azri, A.; Everaus, H.; Gerber, E.; Gomez, J.G.; Pettersson, B.G.; Soga, Y.; et al. Systematic Review of Cytokines and Growth Factors for the Management of Oral Mucositis in Cancer Patients. Support. Care Cancer 2013, 21, 343–355. [Google Scholar] [CrossRef] [Green Version]
- Gharaee-Kermani, M.; Phan, S.H. Role of Cytokines and Cytokine Therapy in Wound Healing and Fibrotic Diseases. Curr. Pharm. Des. 2001, 7, 1083–1103. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Bekoe, E.O.; Boakye, Y.D.; Dapaah, S.O.; Appiah, T.; Bekoe, O.S. Medicinal Plants and Natural Products with Demonstrated Wound Healing Properties. In Wound Healing—New Insights into Ancient Challenges; Alexandrescu, V.A., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Al Wahbi, A. Autoamputation of Diabetic Toe with Dry Gangrene: A Myth or a Fact? Diabetes Metab. Syndr. Obes. 2018, 11, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A Review of Potential Therapeutic Effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Petrovska, B.B.; Cekovska, S. Extracts from the History and Medical Properties of Garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.; Lis-Balchin, M.; Kirk-Smith, M. Evaluation of Massage with Essential Oils on Childhood Atopic Eczema. Phytother. Res. 2000, 14, 452–456. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The Antibacterial Activity of Lavender Essential Oil Alone and in Combination with Octenidine Dihydrochloride Against MRSA Strains. Molecules 2019, 25, 95. [Google Scholar] [CrossRef] [Green Version]
- Peterson, L.R. Currently Available Antimicrobial Agents and their Potential for use as Monotherapy. Clin. Microbiol. Infect. 2008, 14, 30–45. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial Activity and Mechanism of Linalool Against Pseudomonas Aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Buton, N.; Badea, M.L.; Marutescu, L. Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Sarkar, P.; Arockiaraj, J.; Stefi, R.; Pasupuleti, M.; Paray, B.; Al-Sadoon, M.A. Antioxidant Molecular Mechanism Of adenosyl Homocysteinase From cyanobacteria and its Wound Healing Process in Fibroblast Cells. Mol. Biol. Rep. 2020, 47, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Shroff, A.; Mamalis, A.; Jagdeo, J. Oxidative Stress and Skin Fibrosis. Curr. Pathobiol. Rep. 2014, 2, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Senoo, H.; Umezono, Y.; Sanada, C.; Barikmo, I.; Berhe, N.; et al. The Total Antioxidant Content of More than 3100 Foods, Beverages, Spices, Herbs and Supplements used Worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Fereydouni, N.; Darroudi, M.; Movaffagh, J.; Shahroodi, A.; Butler, A.E.; Ganjali, S.; Sahebkar, A. Curcumin Nanofibers for the Purpose of Wound Healing. J. Cell. Physiol. 2019, 234, 5537–5554. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, B.; Vadalà, M.; Laurino, C. Nutrition in Wound Healing: Investigation of the Molecular Mechanisms, a Narrative Review. J. Wound Care 2019, 28, 683–693. [Google Scholar] [CrossRef]
- Callus, C.A.; Vella, S.; Ferry, P. Scurvy is Back. Nutr. Metab. Insights 2018, 11, 1178638818809097. [Google Scholar] [CrossRef] [Green Version]
- Gunes Bilgili, S.; Calka, O.; Akdeniz, N.; Bayram, I.; Metin, A. The Effects of Retinoids on Secondary Wound Healing: Biometrical and Histopathological Study in Rats. J. Dermatol. Treat. 2013, 24, 283–289. [Google Scholar] [CrossRef]
- Shekhar, C. An Innovative Technique in Local Antibiotic Delivery Method in Open Infected Wounds of the Musculoskeletal System. Int. J. Low Extrem. Wounds 2019, 18, 153–160. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.; Craciunescu, O. Natural Composite Dressings Based on Collagen, Gelatin and Plant Bioactive Compounds for Wound Healing: A Review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing from Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakaria, A.; Labib, O. Evaluation of Emissions from Medical Waste Incinerators in Alexandria. J. Egypt Public Health Assoc. 2003, 78, 225–244. [Google Scholar]
- Yao, C.; Yao, P.; Wu, H.; Zha, Z. Acceleration of Wound Healing in Traumatic Ulcers by Absorbable Collagen Sponge Containing Recombinant Basic Fibroblast Growth Factor. Biomed. Mater. 2006, 1, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Catanzano, O.; Quaglia, F.; Boateng, J.S. Wound Dressings as Growth Factor Delivery Platforms for Chronic Wound Healing. Expert Opin. Drug Deliv. 2021, 18, 737–759. [Google Scholar] [CrossRef] [PubMed]
- Vivcharenko, V.; Przekora, A. Modifications of Wound Dressings with Bioactive Agents to Achieve Improved Pro-Healing Properties. Appl. Sci. 2021, 11, 4114. [Google Scholar] [CrossRef]
- Mohandas, A.; PT, S.K.; Raja, B.; Lakshmanan, V.; Jayakumar, R. Exploration of Alginate Hydrogel/Nano Zinc Oxide Composite Bandages for Infected Wounds. Int. J. Nanomed. 2015, 10, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Pachuau, L. Recent Developments in Novel Drug Delivery Systems for Wound Healing. Exp. Opin. Drug Deliv. 2015, 12, 1895–1909. [Google Scholar] [CrossRef]
- Ardekani, N.T.; Khorrama, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of Electrospun Poly (Vinyl Alcohol)-Based Nanofiber Mats Incorporated with Zataria Multiflora Essential Oil as Potential Wound Dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef]
- Garcia-Salinas, S.; Evangelopoulos, M.; Gamez-Herrera, E.; Arrueboa, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun Anti-Inflammatory Patch Loaded with Essential Oils for Wound Healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef]
- Bullough, L.; Johnson, S.; Forder, R. Evaluation of a Foam Dressing for Acute and Chronic Wound Exudate Management. Br. J. Community Nurs. 2015, 20, S17–S24. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Ashouri, F.; Beyranvand, F.; Beigi Boroujeni, N.; Tavafi, M.; Sheikhian, A.; Mohammad Varzi, A.; Shahrokhi, S. Macrophage Polarization in Wound Healing: Role of Aloe Vera/Chitosan Nanohydrogel. Drug Deliv. Transl. Res. 2019, 9, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Wang, J.; Zhao, H.; Yang, X. Self-Assembled Small Molecule Natural Product Gel for Drug Delivery: A Breakthrough in New Application of Small Molecule Natural Products. Acta Pharm. Sin. B 2020, 10, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Lutton, R.E.M.; Moore, J.; Larrañeta, E.; Donnelly, R.F. Microneedle Characterisation: The Need for Universal Acceptance Criteria and GMP Specifications when Moving Towards Commercialisation. Drug Deliv. Transl. Res. 2016, 5, 313–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarah, S.; Sharma, M.; Wen, J. Recent Advances in Microneedle-Based Drug Delivery: Special Emphasis on its use in Paediatric Population. Eur. J. Pharm. Biopharm. 2019, 136, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and Angiogenic Chitosan Microneedle Array Patch for Promoting Wound Healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Lee, Y.; Kim, G.H.; Park, E.C.; Han, S.H.; Lee, J.G.; Choi, S.; Heo, N.S.; Kim, D.L.; et al. Wound Healing Potential of Antibacterial Microneedles Loaded with Green Tea Extracts. Mater. Sci. Eng. C 2014, 42, 757–762. [Google Scholar] [CrossRef]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, e6847971. [Google Scholar] [CrossRef]
- Liolios, C.C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. Liposomal Incorporation of Carvacrol and Thymol Isolated from the Essential Oil of Origanum Dictamnus, L. and in Vitro Antimicrobial Activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Shoji, Y.; Nakashima, H. Nutraceutics and Delivery Systems. J. Drug Target. 2004, 12, 385–391. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo-Rinhel, A.S.G.; de Andrade, M.F.; Landi-Librandi, A.P.; Azzolini, A.E.C.S.; Kabeya, L.M.; Bastos, J.K.; Lucisano-Valim, Y.M. Incorporation of Baccharis Dracunculifolia DC (Asteraceae) Leaf Extract into Phosphatidylcholine-Cholesterol Liposomes Improves its Anti-Inflammatory Effect in Vivo. Nat. Prod. Res. 2019, 33, 2521–2525. [Google Scholar] [CrossRef]
- Roesken, F.; Uhl, E.; Curri, S.B.; Menger, M.D.; Messmer, K. Acceleration of Wound Healing by Topical Drug Delivery Via Liposomes. Langenbecks Arch. Surg. 2000, 385, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, M.; Wang, H.; Du, S. Increased Cutaneous Wound Healing Effect of Biodegradable Liposomes Containing Madecassoside: Preparation Optimization, in Vitro Dermal Permeation, and in Vivo Bioevaluation. Int. J. Nanomed. 2016, 11, 2995–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an Alternative for Liposomal Delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A Future of Targeted Drug Delivery Systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Shayan, M.; Bourbour, M.; Moghtaderi, M.; Noorbazargan, H.; Eshrati Yeganeh, F.; Saffar, S.; Tahriri, M. Preparation, Optimization and in-Vitro Evaluation of Curcumin-Loaded Niosome@calcium Alginate Nanocarrier as a New Approach for Breast Cancer Treatment. Biology 2021, 10, 173. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, W.; Tsosie, J.K.; Xie, X.; Li, P.; Wan, J.; He, C.; Chen, M. Niosome Encapsulation of Curcumin: Characterization and Cytotoxic Effect on Ovarian Cancer Cells. J. Nanomater. 2016, 2016, e6365295. [Google Scholar] [CrossRef] [Green Version]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, P.; Larijani, B.; Alavi-Moghadam, S.; Tayanloo-Beik, A.; Mohamadi-Jahani, F.; Ranjbaran, N.; Payab, M.; Falahzadeh, K.; Mousavi, M.; Arjmand, B. Mesenchymal Stem Cells-Derived Exosomes for Wound Regeneration. In Cell Biology and Translational Medicine; Turksen, K., Ed.; Springer: Cham, Switzerland, 2018; Volume 4, pp. 119–131. [Google Scholar]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H. A Novel Nanoparticle Drug Delivery System: The Anti-Inflammatory Activity of Curcumin is Enhanced when Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart Nanocarrier-Based Drug Delivery Systems for Cancer Therapy and Toxicity Studies: A Review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Sheorain, J.; Mehra, M.; Thakur, R.; Grewal, S.; Kumari, S. In Vitro Anti-Inflammatory and Antioxidant Potential of Thymol Loaded Bipolymeric (Tragacanth Gum/Chitosan) Nanocarrier. Int. J. Biol. Macromol. 2019, 125, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, A.; Reglero, G. Effects of Thyme Extract Oils (from Thymus Vulgaris, Thymus Zygis, and Thymus Hyemalis) on Cytokine Production and Gene Expression of oxLDL-Stimulated THP-1-Macrophages. J. Obes. 2012, 2012, 104706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh Dastidar, D.; Saha, S.; Chowdhury, M. Porous Microspheres: Synthesis, Characterisation and Applications in Pharmaceutical & Medical Fields. Int. J. Pharm. 2018, 548, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Niu, J.; Chong, Y.; Huang, Y.; Chu, Y.; Xie, S.; Jiang, Z.; Peng, L. Porous Microspheres as Promising Vehicles for the Topical Delivery of Poorly Soluble Asiaticoside Accelerate Wound Healing and Inhibit Scar Formation in Vitro & in Vivo. Eur. J. Pharm. Biopharm. 2016, 109, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M.; He, B.; Gao, B. Intelligent Patches for Wound Management: In Situ Sensing and Treatment. Anal. Chem. 2021, 93, 4687–4696. [Google Scholar] [CrossRef]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.; Mateescu, A.L.; Stan, D. Wound Healing Applications of Creams and “smart” Hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef]
- Ochoa, M.; Rahimi, R.; Zhou, J.; Jiang, H.; Yoon, C.K.; Maddipatla, D.; Narakathu, B.B.; Jain, V.; Oscai, M.M.; Morken, T.J.; et al. Integrated Sensing and Delivery of Oxygen for Next-Generation Smart Wound Dressings. Microsyst. Nanoeng. 2020, 6, 1–16. [Google Scholar] [CrossRef]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart Dressings Based on Nanostructured Fibers Containing Natural Origin Antimicrobial, Anti-Inflammatory, and Regenerative Compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef]
- Tamayol, A.; Hassani Najafabadi, A.; Mostafalu, P.; Yetisen, A.K.; Commotto, M.; Aldhahri, M.; Abdel-wahab, M.S.; Najafabadi, Z.I.; Latifi, S.; Akbari, M.; et al. Biodegradable Elastic Nanofibrous Platforms with Integrated Flexible Heaters for on-Demand Drug Delivery. Sci. Rep. 2017, 7, 9220. [Google Scholar] [CrossRef] [PubMed]
- Mostafalu, P.; Kiaee, G.; Giatsidis, G.; Khalilpour, A.; Nabavinia, M.; Dokmeci, M.R.; Sonkusale, S.; Orgill, D.P.; Tamayol, A.; Khademhosseini, A. A Textile Dressing for Temporal and Dosage Controlled Drug Delivery. Adv. Funct. Mater. 2017, 27, 1702399. [Google Scholar] [CrossRef]
- Cordier, C.; Morton, D.; Murrison, S.; Nelson, A.; O’Leary-Steele, C. Natural Products as an Inspiration in the Diversity-Oriented Synthesis of Bioactive Compound Libraries. Nat. Prod. Rep. 2008, 25, 719–737. [Google Scholar] [CrossRef] [Green Version]
- Meier, B.P.; Lappas, C.M. The Influence of Safety, Efficacy, and Medical Condition Severity on Natural Versus Synthetic Drug Preference. Med. Decis. Mak. 2016, 36, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.; Chambers, E.; Castro, M. What is “Natural”? Consumer Responses to Selected Ingredients. Foods 2018, 7, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pevsic, M. Development of Natural Product Drugs in a Sustainable Manner. In Brief for GSDR; United Nations: San Francisco, CA, USA, 2015. [Google Scholar]
- Choudhary, I. Back to Nature. Nature 2008, 456, 41. [Google Scholar] [CrossRef]
- Oyebode, O.; Kandala, N.; Chilton, P.J.; Lilford, R.J. Use of Traditional Medicine in Middle-Income Countries: A WHO-SAGE Study. Health Policy Plan. 2016, 31, 984–991. [Google Scholar] [CrossRef] [Green Version]
- McNerney, R. Diagnostics for Developing Countries. Diagnostics 2015, 5, 200–209. [Google Scholar] [CrossRef]
- Harris, E. Building Scientific Capacity in Developing Countries. EMBO Rep. 2004, 5, 7–11. [Google Scholar] [CrossRef]
- Li, D.; Martini, N.; Liu, M.; Falconer, J.R.; Locke, M.; Wu, Z.; Wen, J. Non-Ionic Surfactant Vesicles as a Carrier System for Dermal Delivery of (+)-Catechin and their Antioxidant Effects. J. Drug Target. 2021, 29, 310–322. [Google Scholar] [CrossRef]
- Engel, J.B.; Heckler, C.; Tondo, E.C.; Daroit, D.J.; da Silva Malheiros, P. Antimicrobial Activity of Free and Liposome-Encapsulated Thymol and Carvacrol Against Salmonella and Staphylococcus Aureus Adhered to Stainless Steel. Int. J. Food Microbiol. 2017, 252, 18–23. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Kim, M.W.; Kwon, S.; Choi, J.H.; Lee, A. A Promising Biocompatible Platform: Lipid-Based and Bio-Inspired Smart Drug Delivery Systems for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3859. [Google Scholar] [CrossRef] [Green Version]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-Inflammatory Activities Via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated with 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, R.; Yang, J.; Lin, Y.; Lee, W.; Li, J.; Ren, L.; Liu, B.; Jiang, L. Rapidly Fabricated Microneedle Arrays using Magnetorheological Drawing Lithography for Transdermal Drug Delivery. ACS Biomater. Sci. Eng. 2019, 5, 5506–5513. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wong, P.K. A Plasma Lithography Microengineered Assay for Studying Architecture Dependent Wound Healing of Endothelial Cells. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences 2011 MicroTAS, Seattle, WA, USA, 2–6 October 2011; pp. 326–328. [Google Scholar]
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.; Abd Aziz, I.; Fauzi, M.B. Natural 3D-Printed Bioinks for Skin Regeneration and Wound Healing: A Systematic Review. Polymers 2020, 12, 1782. [Google Scholar] [CrossRef] [PubMed]



| Type of Wound Dressing | Features | Limitations | Product Name | References |
|---|---|---|---|---|
| Film dressings | Elastic, durable, comfortable, and conform well to body contours Waterproof and transparent Create a moist healing environment Bacterial and viral barrier Semi-permeable to water vapour and gas | Adhesive films might disrupt newly formed epithelium during dressing change Limited use for highly exuding wounds Might develop leakage channels | Tegaderm™ (3M™, UK Plc.) Opsite Films® (Smith and Nephew) Mepitel®Film (Mölnlycke Health Care Limited) | [32,34,41] |
| Foam dressings | Highly absorbent Easily removable Create a moist healing environment Bacterial and viral barrier Semi-permeable to water vapour and gas | Form an opaque layer, making wound monitoring difficult Limited use for dry wounds Poor stability | Flexsan Biopatch Biatain Cultinova Lyofoam Allevyn Unilene Tielle CuraSpon Kendall Hydrasorb | [33,42] |
| Hydrogel dressings | Create a moist healing environment Pain relief Self-applied or injectable Facilitate autolytic debridement Easily removed | Require a secondary dressing Lack of mechanical strength Inconsistent hydration properties Poor bacterial barrier | Suprasorb® AquaDerm™ Neoheal® Simpurity™ DermaGauze™ Restore | [43,44,45] |
| Bioadhesive dressings | Create a moist healing environment Self-injectable Adhesive | Unremovable Interference with other medical devices Might develop leakage channels | Ligate™ | [37] |
| Mode of Action | Natural Products | References |
|---|---|---|
| Modulators of Cellular Activity | Turmeric, Honey, and E. phaseoloides | [46,47,48,49,50,51,52,53,54,55,56] |
| Modulators of Collagen Synthesis | Aloe vera, A. boonei, C. asiatica | [57,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| Modulators of Angiogenesis | Honey, Aloe Vera, and E. phaseoloides | [59,63,71,72,73,74,75,76] |
| Modulators of the Extracellular Matrix | Honey | [57,77,78,79,80,81] |
| Modulators of Cytokines and Growth Factors | Essential Oils and Honey | [52,53,82,83,84,85,86] |
| Antibiotics and Antimicrobials | Garlic and Lavender | [87,88,89,90] |
| Modulators of Oxidant/Antioxidant Balance | Turmeric and Vanilla | [41,91,92,93] |
| Other | Vitamins A/B/C/D | [94] |
| China | ||
|---|---|---|
| OR (CI) | p-Value | |
| Rural | 6.9 (5.4–8.9) | <0.001 |
| Income quintile | 1.2 (1.1–1.2) | <0.001 |
| Ghana | ||
| OR (CI) | p-Value | |
| Rural | 1.4 (1–2.2) | 0.077 |
| Income quintile | 0.8 (0.7–0.9) | 0.002 |
| India | ||
| OR (CI) | p-Value | |
| Rural | 1.3 (0.9–2) | 0.217 |
| Income quintile | 0.8 (0.7–0.9) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryall, C.; Duarah, S.; Chen, S.; Yu, H.; Wen, J. Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades. Pharmaceutics 2022, 14, 1072. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051072
Ryall C, Duarah S, Chen S, Yu H, Wen J. Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades. Pharmaceutics. 2022; 14(5):1072. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051072
Chicago/Turabian StyleRyall, Cameron, Sanjukta Duarah, Shuo Chen, Haijun Yu, and Jingyuan Wen. 2022. "Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades" Pharmaceutics 14, no. 5: 1072. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14051072