Preparation of Activated Carbon/TiO2 Nanohybrids for Photodegradation of Reactive Red-35 Dye Using Sunlight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AC/TiO2 Nanohybrids
2.3. Characterization of Studied Nanohybrids
2.4. RR-35 Dye Removal Experiments
3. Results and Discussion
3.1. XRD Analysis
3.2. FESEM Analysis
3.3. FTIR Analysis
3.4. TG Analysis
3.5. Effect of AC Content
3.6. Effect of pH
3.7. Effect of Time
3.8. Effect of Initial Concentration of RR-35
3.9. Role of Radical Scavenger
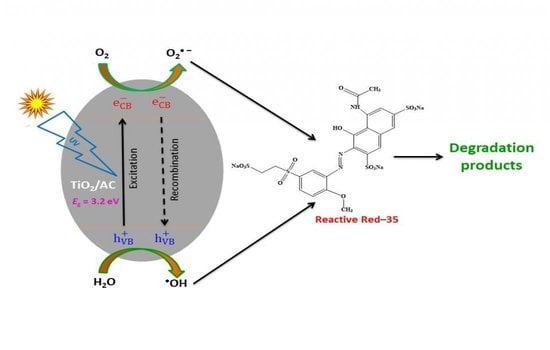
3.10. Photodegradation Mechanism
3.11. Reusability of AC/TiO2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Yang, Z. Industrial water pollution, water environment treatment, and health risks in China. Environ. Pollut. 2016, 218, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Alves de Lima, R.O.; Bazo, A.P.; Salvadori, D.M.F.; Rech, C.M.; de Palma Oliveira, D.; de AragãoUmbuzeiro, G. Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat. Res. 2007, 626, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.V.; Murthy, D.V.S. Statistical optimization for decolorization of textile dyes using Trametes versicolor. J. Hazard. Mater. 2009, 165, 909–914. [Google Scholar] [CrossRef]
- Mezohegyi, G.; van der Zee, F.P.; Font, J.A.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Ike, I.A.; Lee, Y.; Hur, J. Impacts of advanced oxidation processes on disinfection byproducts from dissolved organic matter upon post-chlor(am)ination: A critical review. Chem. Eng. J. 2019, 375, 121929. [Google Scholar] [CrossRef]
- Sarker, M.; Bhadra, B.N.; Seo, P.W.; Jhung, S.H. Adsorption of benzotriazole and benzimidazole from water over a Co-based metal azolate framework MAF-5(Co). J. Hazard. Mater. 2017, 324, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Isa, M.H. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Saravanan, R.; Ravichandran, K.; Gracia, F.; Agarwal, S.; Gupta, V.K. Synthesis and characterization of metal oxides (CeO2, CuO, NiO, Mn3O4, SnO2 and ZnO) nanoparticles as photo catalysts for degradation of textile dyes. J. Photochem. Photobiol. B 2017, 173, 43–49. [Google Scholar] [CrossRef]
- Al-Amin, M.; Dey, S.C.; Rashid, T.U.; Ashaduzzaman, M.; Shamsuddin, S.M. Solar assisted photocatalytic degradation of reactive azo dyes in presence of anatase titanium dioxide. Int. J. Latest Res. Eng. Technol. 2016, 2, 14–21. [Google Scholar]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Paul, S.C.; Dey, S.C.; Molla, M.A.I.; Islam, M.S.; Debnath, S.; Miah, M.Y.; Ashaduzzaman, M.; Sarker, M. Nanomaterials as electrocatalyst for hydrogen and oxygen evolution reaction: Exploitation of challenges and current progressions. Polyhedron 2021, 193, 114871. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent progress in enhancing photocatalytic efficiency of TiO2-based materials. Appl. Catal. A Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Asiltürk, M.; Şener, Ş. TiO2-activated carbon photocatalysts: Preparation, characterization and photocatalytic activities. Chem. Eng. J. 2012, 180, 354–363. [Google Scholar] [CrossRef]
- Hasan, A.K.M.M.; Dey, S.C.; Rahman, M.M.; Zakaria, A.M.; Sarker, M.; Ashaduzzaman, M.; Shamsuddin, S.M. A kaolinite/TiO2/ZnO-based novel ternary composite for photocatalytic degradation of anionic azo dyes. Bull. Mater. Sci. 2020, 43, 1–9. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, X.; Liu, W. The synthesis strategies and photocatalytic performances of TiO2/MOFs composites: A state-of-the-art review. Chem. Eng. J. 2020, 391, 123601. [Google Scholar] [CrossRef]
- Sun, Z.; Bai, C.; Zheng, S.; Yang, X.; Frost, R.L. A comparative study of different porous amorphous silica minerals supported TiO2 catalysts. Appl. Catal. A Gen. 2013, 458, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Suba, V.; Saravanabhavan, M.; Krishna, L.S.; Kaleemulla, S.; Kumar, E.R.; Rathika, G. Evaluation of curcumin assistance in the antimicrobial and photocatalytic activity of a carbon based TiO2 nanocomposite. New J. Chem. 2020, 44, 15895–15907. [Google Scholar] [CrossRef]
- Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Souza, J.R.B.; Zhang, T.; Pilau, E.J.; Asefa, T.; Almeida, V.C. Sol-gel synthesis of new TiO2/activated carbon photocatalyst and its application for degradation of tetracycline. Ceram. Int. 2017, 43, 4411–4418. [Google Scholar] [CrossRef]
- Liu, S.X.; Chen, X.Y.; Chen, X. A TiO2/AC composite photocatalyst with high activity and easy separation prepared by a hydrothermal method. J. Hazard. Mat. 2007, 143, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, Z.; Bala´zs, N.; Alapi, T.; Wittmann, G.; Sipos, P.; Dombi, A.; Mogyoro´si, K. Synthesis, structure and photocatalytic properties of Fe(III)-doped TiO2 prepared from TiCl3. Appl. Catal. B. Environ. 2008, 81, 27–37. [Google Scholar] [CrossRef]
- Bansal, P.; Sud, D. Photocatalytic degradation of commercial dye, CI Reactive Red 35 in aqueous suspension: Degradation pathway and identification of intermediates by LC/MS. J. Mol. Catal. A Chem. 2013, 374–375, 66–72. [Google Scholar] [CrossRef]
- Sarker, M.; Ahmed, I.; Jhung, S.H. Adsorptive removal of herbicides from water over nitrogen-doped carbon obtained from ionic liquid@ZIF-8. Chem. Eng. J. 2017, 323, 203–211. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, A.; Zhong, S.; Liu, F.; Zhang, Q. Preparation and characterization of polymer-based spherical activated carbons with tailored pore structure. J. Appl. Polym. Sci. 2008, 109, 1692–1698. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.; Hong, J.; Sun, C. Low-temperature preparation and microwave photocatalytic activity study of TiO2-mounted activated carbon. J. Hazard. Mater. 2007, 142, 208–215. [Google Scholar] [CrossRef]
- Huang, D.; Miyamoto, Y.; Matsumoto, T.; Tojo, T.; Fan, T.; Ding, J.; Guo, Q.; Zhang, D. Preparation and characterization of high-surface-area TiO2/activated carbon by low-temperature impregnation. Sep. Purif. Technol. 2011, 78, 9–15. [Google Scholar] [CrossRef]
- Xing, B.; Shi, C.; Zhang, C.; Yi, G.; Chen, L.; Guo, H.; Huang, G.; Cao, J. Preparation of TiO2/activated carbon composites for photocatalytic degradation of RhB under UV light irradiation. J. Nanomater. 2016, 2016, 8393648. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-J.; Zhuang, Y.; Fu, X.Z. New insight for enhanced photocatalytic activity of TiO2 by doping carbon nanotubes: A case study on degradation of benzene and methyl orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Anyika, C.; Asri, N.A.M.; Majid, Z.A.; Yahya, A.; Jaafar, J. Synthesis and characterization of magnetic activated carbon developed from palm kernel shells. Nanotechnol. Environ. Eng. 2017, 2, 1–25. [Google Scholar] [CrossRef]
- Saravanan, R.; Aviles, J.; Gracia, F.; Mosquera, E.; Gupta, V.K. Crystallinity and lowering band gap induced visible light photocatalytic activity of TiO2/CS (Chitosan) nanocomposites. Int. J. Biol. Macromol 2018, 109, 1239–1245. [Google Scholar] [CrossRef]
- Xue, G.; Liu, H.H.; Chen, Q.Y.; Hills, C.; Tyrer, M.; Innocent, F. Synergy between surface adsorption and photocatalysis during degradation of humic acid on TiO2/activated carbon composites. J. Hazard. Mater. 2011, 186, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.C.; Moztahida, M.; Sarker, M.; Ashaduzzaman, M.; Shamsuddin, S.M. pH-triggered interfacial interaction of kaolinite/chitosan nanocomposites with anionic azo dye. J. Compos. Sci. 2019, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Atout, H.; Bouguettoucha, A.; Chebli, D.; Gatica, J.M.; Vidal, H.; Yeste, M.P.; Amrane, A. Integration of adsorption and photocatalytic degradation of methylene blue using TiO2 supported on granular activated carbon. Arab. J. Sci. Eng. 2017, 42, 1475–1486. [Google Scholar] [CrossRef]
- Sakib, A.A.M.; Masum, S.M.; Hoinkis, J.; Islam, R.; Molla, M.A.I. Synthesis of CuO/ZnO nanocomposites and their application in photodegradation of toxic textile dye. J. Compos. Sci. 2019, 3, 91. [Google Scholar] [CrossRef] [Green Version]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Ahsan, S.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Degradation, kinetics, and mineralization in the solar photocatalytic treatment of aqueous amitrole solution with titanium dioxide. Environ. Eng. Sci. 2018, 35, 401–407. [Google Scholar] [CrossRef]
- Santhosh, C.; Malathi, A.; Daneshvar, E.; Kollu, P.; Bhatnagar, A. Photocatalytic degradation of toxic aquatic pollutants by novel magnetic 3D-TiO2@HPGA nanocomposite. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Molla, M.A.I.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Fabrication of Ag-doped ZnO by mechanochemical combustion method and their application into photocatalytic Famotidine degradation. J. Environ. Sci. Health. Part A 2019, 54, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhu, L.; Geng, P.; Chen, G. Self-assembly graphitic carbon nitride quantum dots anchored on TiO2 nanotube arrays: An efficient heterojunction for pollutants degradation under solar light. J. Hazard. Mater. 2016, 316, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Omri, A.; Benzina, M. Almond shell activated carbon: Adsorbent and catalytic support in the phenol degradation. Environ. Monit. Assess. 2014, 186, 3875–3890. [Google Scholar] [CrossRef] [PubMed]
- Baca, M.; Wenelska, K.; Mijowska, E.; Kaleńczuk, R.J.; Zielińska, B. Physicochemical and photocatalytic characterization of mesoporous carbon/titanium dioxide spheres. Diam. Relat. Mater. 2020, 101, 107551. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Evaluation of reaction mechanism for photocatalytic degradation of dye with self-sensitized TiO2 under visible light irradiation. Open J. Inorg. Non-Met. Mater. 2017, 7, 1–7. [Google Scholar]
- Ahmed, A.Z.; Islam, M.M.; Islam, M.M.; Masum, S.M.; Islam, R.; Molla, M.A.I. Fabrication and characterization of B/Sn-doped ZnO nanoparticles via mechanochemical method for photocatalytic degradation of rhodamine B. Inorg. Nano-Met. Chem. 2020. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Lourenc¸o, V.S.; Carvalho, W.A. Removal of phenol in seawater by heterogeneous photocatalysis using activated carbon materials modified with TiO2. Catal. Today 2020. [Google Scholar] [CrossRef]
- Yao, S.; Li, J.; Shi, Z. Immobilization of TiO2 nanoparticles on activated carbon fiber and its photodegradation performance for organic pollutants. Particuology 2010, 8, 272–278. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondol, B.; Sarker, A.; Shareque, A.M.; Dey, S.C.; Islam, M.T.; Das, A.K.; Shamsuddin, S.M.; Molla, M.A.I.; Sarker, M. Preparation of Activated Carbon/TiO2 Nanohybrids for Photodegradation of Reactive Red-35 Dye Using Sunlight. Photochem 2021, 1, 54-66. https://0-doi-org.brum.beds.ac.uk/10.3390/photochem1010006
Mondol B, Sarker A, Shareque AM, Dey SC, Islam MT, Das AK, Shamsuddin SM, Molla MAI, Sarker M. Preparation of Activated Carbon/TiO2 Nanohybrids for Photodegradation of Reactive Red-35 Dye Using Sunlight. Photochem. 2021; 1(1):54-66. https://0-doi-org.brum.beds.ac.uk/10.3390/photochem1010006
Chicago/Turabian StyleMondol, Bappy, Anupam Sarker, A. M. Shareque, Shaikat Chandra Dey, Mohammad Tariqul Islam, Ajoy Kumar Das, Sayed Md. Shamsuddin, Md. Ashraful Islam Molla, and Mithun Sarker. 2021. "Preparation of Activated Carbon/TiO2 Nanohybrids for Photodegradation of Reactive Red-35 Dye Using Sunlight" Photochem 1, no. 1: 54-66. https://0-doi-org.brum.beds.ac.uk/10.3390/photochem1010006