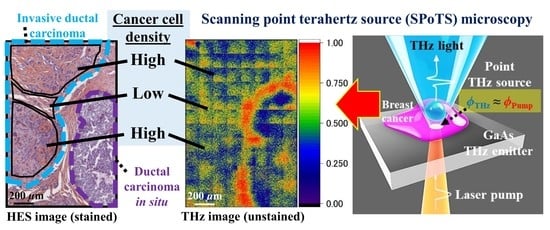
Label-Free Observation of Micrometric Inhomogeneity of Human Breast Cancer Cell Density Using Terahertz Near-Field Microscopy
Abstract
:1. Introduction
2. Setup of Terahertz Near-Field Microscopy System
3. Sample Information
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Soerjomataram, I. The Changing Global Burden of Cancer: Transitions in Human Development and Implications for Cancer Prevention and Control. In Cancer: Disease Control Priorities, 3rd ed.; Gelband, H., Jha, P., Sankaranarayanan, R., Horton, S., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2015; Volume 3. [Google Scholar]
- Meyer, T.; Schmitt, M.; Guntinas-Lichius, O.; Popp, J. Toward an all-optical biopsy. Opt. Photonics News 2019, 30, 26–33. [Google Scholar] [CrossRef]
- Son, J.H.; Oh, S.J.; Cheon, H. Potential clinical applications of terahertz radiation. J. Appl. Phys. 2019, 125, 190901. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, S.H.; Ji, Y.B.; Jeong, K.; Park, Y.; Yang, J.; Park, D.W.; Noh, S.K.; Kang, S.G.; Huh, Y.M.; et al. Study of freshly excised brain tissues using terahertz imaging. Biomed. Opt. Express 2014, 5, 2837–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolyanskaya, O.; Chernomyrdin, N.; Konovko, A.; Zaytsev, K.; Ozheredov, I.; Cherkasova, O.; Nazarov, M.; Guillet, J.-P.; Kozlov, S.; Kistenev, Y.; et al. Terahertz biophotonics as a tool for studies of dielectric and spectral properties of biological tissues and liquids. Prog. Quantum Electron. 2018, 62, 1–77. [Google Scholar] [CrossRef]
- McIntyre, G.I. Cell hydration as the primary factor in carcinogenesis: A unifying concept. Med. Hypotheses 2006, 66, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Özer, Z.; Gök, S.; Altan, H.; Severcan, F. Concentration-based measurement studies of L-tryptophan using terahertz time-domain spectroscopy (THz-TDS). Appl. Spectrosc. 2014, 68, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Yang, H.J.; Lee, S.H.; Kim, Y.A.; Son, J.H. Terahertz molecular resonance of cancer DNA. Sci. Rep. 2016, 6, 37103. [Google Scholar] [CrossRef] [Green Version]
- Cheon, H.; Yang, H.J.; Son, J.H. Toward clinical cancer imaging using terahertz spectroscopy. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1–9. [Google Scholar] [CrossRef]
- Chernomyrdin, N.V.; Kucheryavenko, A.S.; Kolontaeva, G.S.; Katyba, G.M.; Karalkin, P.A.; Parfenov, V.A.; Gryadunova, A.A.; Norkin, N.E.; Smolyanskaya, O.A.; Minin, O.A.; et al. A potential of terahertz solid immersion microscopy for visualizing sub-wavelength-scale tissue spheroids. Proc. SPIE 2018, 10677, 106771Y. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/10677/2306132/A-potential-of-terahertz-solid-immersion-microscopy-for-visualizing-sub/10.1117/12.2306132.short?SSO=1 (accessed on 19 April 2021).
- Grootendorst, M.R.; Fitzgerald, A.J.; Brouwer de Koning, S.G.; Santaolalla, A.; Portieri, A.; Van Hemelrijck, M.; Young, M.R.; Owen, J.; Cariati, M.; Pepper, M.; et al. Use of a handheld terahertz pulsed imaging device to differentiate benign and malignant breast tissue. Biomed. Opt. Express 2017, 8, 2932–2945. [Google Scholar] [CrossRef] [Green Version]
- Cassar, Q.; Caravera, S.; MacGrogan, G.; Bücher, T.; Hillger, P.; Pfeiffer, U.; Zimmer, T.; Guillet, J.P.; Mounaix, P. Terahertz refractive index-based morphological dilation for breast carcinoma delineation. Sci. Rep. 2021, 11, 6457. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.S.; Patel, R.; Neel, V.A.; Giles, R.H.; Yaroslavsky, A.N. Imaging of ex vivo nonmelanoma skin cancers in the optical and terahertz spectral regions optical and terahertz skin cancers imaging. J. Biophotonics 2014, 7, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.C.; Park, J.Y.; Ahn, K.M.; Park, C.; Son, J.H. Terahertz imaging of excised oral cancer at frozen temperature. Biomed. Opt. Express 2013, 4, 1413–1421. [Google Scholar] [CrossRef]
- Ji, Y.B.; Park, C.H.; Kim, H.; Kim, S.H.; Lee, G.M.; Noh, S.K.; Jeon, T.I.; Son, J.H.; Huh, Y.M.; Haam, S.; et al. Feasibility of terahertz reflectometry for discrimination of human early gastric cancers. Biomed. Opt. Express 2015, 6, 1398–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.B.; Oh, S.J.; Kang, S.G.; Heo, J.; Kim, S.H.; Choi, Y.; Song, S.; Son, H.Y.; Kim, S.H.; Lee, J.H.; et al. Terahertz reflectometry imaging for low and high grade gliomas. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, T.C.; El-Shenawee, M.; Campbell, L.K. Terahertz imaging of excised breast tumor tissue on paraffin sections. IEEE Trans. Antennas Propag. 2015, 63, 2088–2097. [Google Scholar] [CrossRef]
- Barr, L.E.; Karlsen, P.; Hornett, S.M.; Hooper, I.R.; Mrnka, M.; Lawrence, C.R.; Phillips, D.B.; Hendry, E. Super-resolution imaging for sub-IR frequencies based on total internal reflection. Optica 2021, 8, 88–94. [Google Scholar] [CrossRef]
- Adam, A.J.L.; Brok, J.M.; Seo, M.A.; Ahn, K.J.; Kim, D.S.; Kang, J.H.; Park, Q.H.; Nagel, M.; Planken, P.C.M. Advanced terahertz electric near-field measurements at sub-wavelength diameter metallic apertures. Opt. Express 2008, 16, 7407–7417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Tang, D.; Hu, J.; Tang, M.; Zhang, M.; Cui, H.L.; Wang, L.; Chang, C.; Fan, C.; Li, J. Near-Field Nanoscopic Terahertz Imaging of Single Proteins. Small 2021, 17, 2005814. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Pham, H.H.; Hisatake, S.; Minin, O.V.; Nagatsuma, T.; Minin, I.V. Enhancement of spatial resolution of terahertz imaging systems based on terajet generation by dielectric cube. APL Photonics 2017, 2, 056106. [Google Scholar] [CrossRef] [Green Version]
- Awad, M.; Nagel, M.; Kurz, H. Tapered Sommerfeld wire terahertz near-field imaging. Appl. Phys. Lett. 2009, 94, 051107. [Google Scholar] [CrossRef]
- Blanchard, F.; Doi, A.; Tanaka, T.; Hirori, H.; Tanaka, H.; Kadoya, Y.; Tanaka, K. Real-time terahertz near-field microscope. Opt. Express 2011, 19, 8277–8284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Yan, S.; Zang, Z.; Geng, G.; Yang, Z.; Li, J.; Wang, L.; Yao, C.; Cui, H.L.; Chang, C. Single cell imaging with near-field terahertz scanning microscopy. Cell Prolif. 2020, 53, e12788. [Google Scholar] [CrossRef] [PubMed]
- Serita, K.; Mizuno, S.; Murakami, H.; Kawayama, I.; Takahashi, Y.; Yoshimura, M.; Mori, Y.; Darmo, J.; Tonouchi, M. Scanning laser terahertz near-field imaging system. Opt. Express 2012, 20, 12959–12965. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Serita, K.; Zang, Z.; Murakami, H.; Kawayama, I.; Cassar, Q.; MacGrogan, G.; Guillet, J.P.; Mounaix, P.; Tonouchi, M. Scanning laser terahertz near-field reflection imaging system. Appl. Phys. Express 2019, 12, 122005. [Google Scholar] [CrossRef]
- Serita, K.; Murakami, H.; Kawayama, I.; Takahashi, Y.; Yoshimura, M.; Mori, Y.; Tonouchi, M. Evaluation of human hairs with terahertz wave. Opt. Eng. 2013, 53, 031205. [Google Scholar] [CrossRef]
- Murakami, H.; Serita, K.; Maekawa, Y.; Fujiwara, S.; Matsuda, E.; Kim, S.; Kawayama, I.; Tonouchi, M. Scanning laser THz imaging system. J. Phys. D Appl. Phys. 2014, 47, 374007. [Google Scholar] [CrossRef]
- Serita, K.; Matsuda, E.; Okada, K.; Murakami, H.; Kawayama, I.; Tonouchi, M. Invited Article: Terahertz microfluidic chips sensitivity-enhanced with a few arrays of meta-atoms. APL Photonics 2018, 3, 051603. [Google Scholar] [CrossRef] [Green Version]
- Serita, K.; Murakami, H.; Kawayama, I.; Tonouchi, M. A terahertz-microfluidic chip with a few arrays of asymmetric meta-atoms for the ultra-trace sensing of solutions. Photonics 2019, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Serita, K.; Cassar, Q.; Murakami, H.; MacGrogan, G.; Guillet, J.P.; Mounaix, P.; Tonouchi, M. Terahertz near-field microscopy of ductal carcinoma in situ (DCIS) of the breast. J. Phys. Photonics 2020, 2, 044008. [Google Scholar] [CrossRef]
- Bowman, T.; El-Shenawee, M.; Campbell, L.K. Terahertz transmission vs reflection imaging and model-based characterization for excised breast carcinomas. Biomed. Opt. Express 2016, 7, 3756–3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhong, S.; Zhang, J.; Ding, J.; Liu, Z.; Huang, Y.; Zhou, N.; Nsengiyumva, W.; Zhang, T. Application of Terahertz Spectroscopy and Imaging in the Diagnosis of Prostate Cancer. Curr. Opt. Photonics 2020, 4, 31–43. [Google Scholar]
- Wahaia, F.; Valusis, G.; Bernardo, L.M.; Almeida, A.; Moreira, J.A.; Lopes, P.C.; Macutkevic, J.; Kasalynas, I.; Seliuta, D.; Adomavicius, R. Detection of colon cancer by terahertz techniques. J. Mol. Struct. 2011, 1006, 77–82. [Google Scholar] [CrossRef]
- Nagai, M.; Tanaka, K.; Ohtake, H.; Bessho, T.; Sugiura, T.; Hirosumi, T.; Yoshida, M. Generation and detection of terahertz radiation by electro-optical process in GaAs using 1.56 μm fiber laser pulses. Appl. Phys. Lett. 2004, 85, 3974–3976. [Google Scholar] [CrossRef]
- Fumeaux, C.; Lin, H.; Serita, K.; Withayachumnankul, W.; Kaufmann, T.; Tonouchi, M.; Abbott, D. Distributed source model for the full-wave electromagnetic simulation of nonlinear terahertz generation. Opt. Express 2012, 20, 18397–18414. [Google Scholar] [CrossRef] [Green Version]
- Xin, X.; Altan, H.; Saint, A.; Matten, D.; Alfano, R.R. Terahertz absorption spectrum of para and ortho water vapors at different humidities at room temperature. J. Appl. Phys. 2006, 100, 094905. [Google Scholar] [CrossRef]
- Doleshal, M.; Magotra, A.A.; Choudhury, B.; Cannon, B.D.; Labourier, E.; Szafranska, A.E. Evaluation and validation of total RNA extraction methods for microRNA expression analyses in formalin-fixed, paraffin-embedded tissues. J. Mol. Diagnostics 2008, 10, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Kong, N.S.P.; Ibrahim, H. Color image enhancement using brightness preserving dynamic histogram equalization. IEEE Trans. Consum. Electron. 2008, 54, 1962–1968. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Fukushi, Y.; Kubota, O.; Itsuji, T.; Ouchi, T.; Yamamoto, S. Origin and quantification of differences between normal and tumor tissues observed by terahertz spectroscopy. Phys. Med. Biol. 2016, 61, 6808. [Google Scholar] [CrossRef] [PubMed]
- Cassar, Q.; Al-Ibadi, A.; Mavarani, L.; Hillger, P.; Grzyb, J.; MacGrogan, G.; Zimmer, T.; Pfeiffer, U.R.; Guillet, J.P.; Mounaix, P. Pilot study of freshly excised breast tissue response in the 300–600 GHz range. Biomed. Opt. Express 2018, 9, 2930–2942. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, K.; Cassar, Q.; Murakami, H.; MacGrogan, G.; Guillet, J.-P.; Mounaix, P.; Tonouchi, M.; Serita, K. Label-Free Observation of Micrometric Inhomogeneity of Human Breast Cancer Cell Density Using Terahertz Near-Field Microscopy. Photonics 2021, 8, 151. https://0-doi-org.brum.beds.ac.uk/10.3390/photonics8050151
Okada K, Cassar Q, Murakami H, MacGrogan G, Guillet J-P, Mounaix P, Tonouchi M, Serita K. Label-Free Observation of Micrometric Inhomogeneity of Human Breast Cancer Cell Density Using Terahertz Near-Field Microscopy. Photonics. 2021; 8(5):151. https://0-doi-org.brum.beds.ac.uk/10.3390/photonics8050151
Chicago/Turabian StyleOkada, Kosuke, Quentin Cassar, Hironaru Murakami, Gaëtan MacGrogan, Jean-Paul Guillet, Patrick Mounaix, Masayoshi Tonouchi, and Kazunori Serita. 2021. "Label-Free Observation of Micrometric Inhomogeneity of Human Breast Cancer Cell Density Using Terahertz Near-Field Microscopy" Photonics 8, no. 5: 151. https://0-doi-org.brum.beds.ac.uk/10.3390/photonics8050151