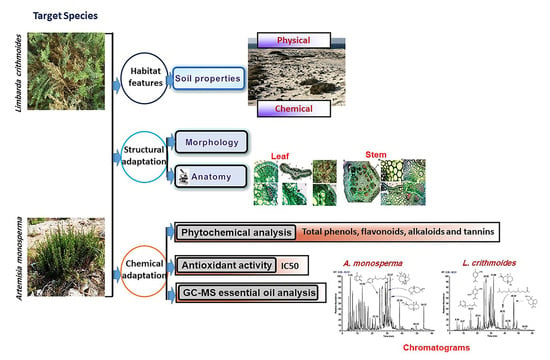
Structural and Chemical Adaptations of Artemisia monosperma Delile and Limbarda crithmoides (L.) Dumort. in Response to Arid Coastal Environments along the Mediterranean Coast of Egypt
Abstract
:1. Introduction
2. Results
2.1. Soil Properties
2.2. Morphological and Anatomical Features of A. monosperma and L. crithmoides
2.3. Phytochemical Analysis of A. monosperma and L. crithmoides
2.3.1. Secondary Metabolites
2.3.2. Antioxidant Activity
2.3.3. Essential Oils Characterization
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Soil Sampling and Analysis
4.3. Plant Samples Collection and Analysis
4.3.1. Samples Collection
4.3.2. Morphological and Anatomical Features
4.3.3. Phytochemicals Analysis
4.3.4. Antioxidant Activity by Free Radical Scavenging Method
4.3.5. Essential Oil Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zahran, M.A.; Willis, A.J. The Vegetation of Egypt, 2nd ed.; Springer: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Carboni, M.; Santoro, R.; Acosta, A.T.R. Dealing with Scarce Data to Understand How Environmental Gradients and Propagule Pressure Shape Fine-Scale Alien Distribution Patterns on Coastal Dunes. J. Veg. Sci. 2011, 22, 751–765. [Google Scholar] [CrossRef]
- Ciccarelli, D. Mediterranean Coastal Sand Dune Vegetation: Influence of Natural and Anthropogenic Factors. Environ. Manag. 2014, 54, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.; Fois, M.; Fenu, G. The Influence of Natural and Anthropogenic Factors on the Floristic Features of the Northern Coast Nile Delta in Egypt. Plant Biosyst. 2018, 152, 407–415. [Google Scholar] [CrossRef]
- Abdelaal, M.; Ahmed, D.; Fois, M.; Fenu, G.; Bacchetta, G. Floristic Patterns and Ecological Drivers of Sand Dune Ecosystem along the Mediterranean Coast of Egypt. Arid Land Res. Manag. 2019, 33, 388–411. [Google Scholar] [CrossRef]
- Ciccarelli, D. Mediterranean Coastal Dune Vegetation: Are Disturbance and Stress the Key Selective Forces That Drive the Psammophilous Succession? Estuar. Coast. Shelf Sci. 2015, 165, 247–253. [Google Scholar] [CrossRef]
- Bar Kutiel, P.; Katz, O.; Ziso-Cohen, V.; Divinsky, I.; Katra, I. Water Availability in Sand Dunes and Its Implications for the Distribution of Artemisia monosperma. Catena 2016, 137, 144–151. [Google Scholar] [CrossRef]
- Ungar, I.A. Are Biotic Factors Significant in Influencing the Distribution of Halophytes in Saline Habitats? Bot. Rev. 1998, 64, 176–199. [Google Scholar] [CrossRef]
- Perrone, R.; Salmeri, C.; Brullo, S.; Colombo, P.; De Castro, O. What Do Leaf Anatomy and Micro-Morphology Tell Us about the Psammophilous Pancratium maritimum L. (Amaryllidaceae) in Response to Sand Dune Conditions? Flora Morphol. Distrib. Funct. Ecol. Plants 2015, 213, 20–31. [Google Scholar] [CrossRef]
- Al-Taisan, W.A. The Relation between Phenotypic Plasticity of Senecio glaucus L. and Some Soil Factors. Aust. J. Basic Appl. Sci. 2010, 4, 1369–1375. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Kuster, V.C.; da Silva, L.C.; Meira, R.M.S.A.; Azevedo, A.A. Structural Adaptation and Anatomical Convergence in Stems and Roots of Five Plant Species from a “Restinga” Sand Coastal Plain. Flora Morphol. Distrib. Funct. Ecol. Plants 2018, 243, 77–87. [Google Scholar] [CrossRef]
- Soliman, S.; Mohammad, M.G.; El-Keblawy, A.A.; Omar, H.; Abouleish, M.; Madkour, M.; Elnaggar, A.; Hosni, R.M. Mechanical and Phytochemical Protection Mechanisms of Calligonum comosum in Arid Deserts. PLoS ONE 2018, 13, e0192576. [Google Scholar] [CrossRef] [Green Version]
- Edreva, A.M.; Velikova, V.B.; Tsonev, T.D. Phenylamides in Plants. Russ. J. Plant Physiol. 2007, 54, 287–301. [Google Scholar] [CrossRef]
- Bora, K.S.; Sharma, A. The Genus Artemisia: A Comprehensive Review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [Green Version]
- Hijazi, A.M.; Salhab, A.S. Effects of Artemisia monosperma Ethanolic Leaves Extract on Implantation, Mid-Term Abortion and Parturition of Pregnant Rats. J. Ethnopharmacol. 2010, 128, 446–451. [Google Scholar] [CrossRef]
- Abu-Niaaj, L.; Abu-Zarga, M.; Abdalla, S. Isolation and Inhibitory Effects of Eupatilin, a Flavone Isolated from Artemisia monosperma Del., on Rat Isolated Smooth Muscle. Pharm. Biol. 1996, 34, 134–140. [Google Scholar] [CrossRef]
- Al-Soqeer, A. Antioxidant Activity and Biological Evaluation of Hot-Water Extract of Artemisia monosperma and Capparis spinosa Against Lead Contamination. Res. J. Bot. 2011, 6, 11–20. [Google Scholar] [CrossRef]
- Stavri, M.; Ford, C.H.J.; Bucar, F.; Streit, B.; Hall, M.L.; Williamson, R.T.; Mathew, K.T.; Gibbons, S. Bioactive Constituents of Artemisia monosperma. Phytochemistry 2005, 66, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Stavri, M.; Mathew, K.T.; Gibson, T.; Williamson, R.T.; Gibbons, S. New Constituents of Artemisia monosperma. J. Nat. Prod. 2004, 67, 892–894. [Google Scholar] [CrossRef]
- Hifnawy, M.S.; Abdel Wahab, S.M.; El-Hawary, S.S.; Karawya, M.S. Study of Essential Oil of Artemisia monosperma and Its Larvicidal Effect. Pharm. Biol. 1990, 28, 247–251. [Google Scholar] [CrossRef]
- Zurayk, R.A.; Baalbaki, R. Inula crithmoides: A Candidate Plant for Saline Agriculture. Arid Soil Res. Rehabil. 1996, 10, 213–223. [Google Scholar] [CrossRef]
- Andreani, S.; De Cian, M.C.; Paolini, J.; Desjobert, J.M.; Costa, J.; Muselli, A. Chemical Variability and Antioxidant Activity of Limbarda crithmoides L. Essential Oil from Corsica. Chem. Biodivers. 2013, 10, 2061–2077. [Google Scholar] [CrossRef] [PubMed]
- Adorisio, S.; Giamperi, L.; Ada Bucchini, A.E.; Delfino, D.V.; Marcotullio, M.C. Bioassay-Guided Isolation of Antiproliferative Compounds from Limbarda crithmoides (L.) Dumort. Molecules 2020, 25, 1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucchini, A.; Giamperi, L.; Ricci, D. Total Polyphenol Content, in Vitro Antifungal and Antioxidant Activities of Callus Cultures from Inula crithmoides. Nat. Prod. Commun. 2013, 8, 1587–1590. [Google Scholar] [CrossRef] [Green Version]
- Jallali, I.; Waffo Téguo, P.; Smaoui, A.; Mérillon, J.M.; Abdelly, C.; Ksouri, R. Bio-Guided Fractionation and Characterization of Powerful Antioxidant Compounds from the Halophyte Inula crithmoїdes. Arab. J. Chem. 2020, 13, 2680–2688. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-De-Santayana, M.; Morales, R. Ethnobotanical Review of Wild Edible Plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant Salt-Tolerance Mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Sardans, J.; Peñuelas, J. Plant-Soil Interactions in Mediterranean Forest and Shrublands: Impacts of Climatic Change. Plant Soil 2013, 365. [Google Scholar] [CrossRef] [Green Version]
- Lamalakshmi, D.; Kumar, S.; Basanta Singh, T.; Sharma, S.K.; Beemrote, A.; Devi, C.P.; Chongtham, S.K.; Singh, C.H.; Yumlembam, R.A.; Haribhushan, A.; et al. Adaptation Strategies and Defence Mechanisms of Plants during Environmental Stress. In Medicinal Plants and Environmental Challenges; Springer: Cham, Switzerland, 2017; pp. 359–413. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of Woody Plants to Flooding and Salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Pandey, B.P. Plant Anatomy; S. Chand & Company Ltd.: Ram Nagar, New Delhi, 1996. [Google Scholar]
- Abd Elhalim, M.E.; Abo-Alatta, O.K.; Habib, S.A.; Abd Elbar, O.H. The Anatomical Features of the Desert Halophytes Zygophyllum album L.F. and Nitraria retusa (Forssk.) Asch. Ann. Agric. Sci. 2016, 61, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Abd Elbar, O.H. Development of the Successive Cambia in Sesuvium verrucosum Raf (Aizoaceae). Ann. Agric. Sci. 2015, 60, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Prado, E.; Demarco, D. Laticifers and Secretory Ducts: Similarities and Differences. In Ecosystem Services and Global Ecology; IntechOpen: London, UK, 2018; pp. 103–123. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Evolution of Secondary Metabolites from an Ecological and Molecular Phylogenetic Perspective. Phytochemistry 2003, 3–19. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Konno, K. Latex: A Model for Understanding Mechanisms, Ecology, and Evolution of Plant Defense Against Herbivory. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 311–331. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.; Gulzar, S.; Aziz, I.; Hussain, T.; Gul, B.; Khan, M.A. Effects of Salinity and Ascorbic Acid on Growth, Water Status and Antioxidant System in a Perennial Halophyte. AoB Plants 2015, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selmar, D.; Kleinwächter, M. Influencing the Product Quality by Deliberately Applying Drought Stress during the Cultivation of Medicinal Plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly) Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antiox. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Shukla, S. Impact of Various Factors Responsible for Fluctuation in Plant Secondary Metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of Plant Defense Against Insect Herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holopainen, J.K.; Blande, J.D. Where Do Herbivore-Induced Plant Volatiles Go? Front. Plant Sci. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, D.; Alnaser, O.; Garayev, E.; Baghdikian, B.; Elias, R.; Chiffolleau, P.; Ollivier, E.; Laurent, S.; El Maataoui, M.; Sallanon, H. Ecophysiological and Phytochemical Characterization of Wild Populations of Inula montana L. (Asteraceae) in Southeastern France. Flora Morphol. Distrib. Funct. Ecol. Plants 2017, 236–237, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple Functions of Polyphenols in Plants Inhabiting Unfavorable Mediterranean Areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Balogun, S.O.; Oladosu, I.A.; Liu, Z. Chemical Compositions and Antioxidant Potential of Essential Oils from Leaves and Flowers of Allophylus africanus. J. Essent. Oil-Bear. Plants 2014, 17, 769–775. [Google Scholar] [CrossRef]
- Ishnava, K.B.; Chauhan, J.B.; Barad, M.B. Anticariogenic and Phytochemical Evaluation of Eucalyptus globules Labill. Saudi J. Biol. Sci. 2013, 20, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Saona, C.R.; Polashock, J.; Malo, E.A. Jasmonate-Mediated Induced Volatiles in the American Cranberry, Vaccinium macrocarpon: From Gene Expression to Organismal Interactions. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to Plants and People. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles, D.J.; Simon, J.E. Comparison of Extraction Methods for the Rapid Determination of Essential Oil Content and Composition of Basil. J. Am. Soc. Hortic. Sci. 2019, 115, 458–462. [Google Scholar] [CrossRef] [Green Version]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of Plant Volatiles: Nature’s Diversity and Ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunford, N.T.; Vazquez, R.S. Effect of Water Stress on Plant Growth and Thymol and Carvacrol Concentrations in Mexican Oregano Grown under Controlled Conditions. J. Appl. Hortic. 2005, 7, 20–22. [Google Scholar] [CrossRef]
- Ben Taarit, M.; Msaada, K.; Hosni, K.; Hammami, M.; Kchouk, M.E.; Marzouk, B. Plant Growth, Essential Oil Yield and Composition of Sage (Salvia officinalis L.) Fruits Cultivated under Salt Stress Conditions. Ind. Crops Prod. 2009, 30, 333–337. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Rivas-Sáenz, S.; Penas-Merino, A. Worldwide Bioclimatic Classification System. Glob. Geobot. 2011, 1, 1–638. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region, 3rd ed.; International Centre for Agricultural Research in the Dry Area: Hong Kong, China, 2013. [Google Scholar]
- Crang, R.; Lyons-Sobaski, S.; Wise, R. Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants; Springer: Switzerland, 2018. [Google Scholar]
- Paterson, R.A. Botanical Histochemistry; Principles and Practices; Jensen, W.A., Ed.; Freeman: San Francisco, CA, USA, 1963. [Google Scholar] [CrossRef]
- Peacock, P.; Bradbury, S. Peacock’s Elementary Micro-Technique, 4th ed.; Edward Arnold: London, UK, 1973. [Google Scholar]
- Sadasivam, S.; Manickam, A. Biochemical Methods for Agricultural Science; Wiley Eastern Limited: New Delhi, India, 1992. [Google Scholar]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 2nd ed.; Chapman and Hall: New York, NY, USA, 1984. [Google Scholar]
- Kitts, D.D.; Wijewickreme, A.N.; Hu, C. Antioxidant Properties of a North American Ginseng Extract. Mol. Cell. Biochem. 2000, 203. [Google Scholar] [CrossRef]






| Scheme | Site I | Site II |
|---|---|---|
| Sand (%) | 95.20 ± 1.80 a | 50.72 ± 0.90 b |
| Silt (%) | 3.50 ± 0.85 a | 42.88 ± 1.50 b |
| Clay (%) | 1.20 ± 0.10 a | 6.40 ± 0.30 b |
| Moisture content (MC, %) | 3.5 ± 0.90 a | 4.70 ± 0.08 b |
| Water holding capacity (WHC, %) | 41.30 ± 1.50 a | 62.86 ± 2.30 b |
| pH | 7.81 ± 0.30 a | 8.21 ± 0.50 a |
| Electric conductivity (EC, µS/cm) | 6180 ± 112.0 a | 7730 ± 67.80 b |
| HCO3 (%) | 0.09 ± 0.0 a | 0.12 ± 0.0 a |
| Cl- (%) | 0.64 ± 0.02 a | 1.10 ± 0.06 b |
| SO4- (%) | 0.65 ± 0.05 a | 0.70 ± 0.0 a |
| CaCO3 (%) | 11.0 ± 1.00 a | 7.0 ± 0.50 b |
| Organic carbon (OC, %) | 0.90 ± 0.01 a | 1.40 ± 0.04 b |
| No | A. monosperma Compounds | RT | MW | MF | Concentration% | L. crithmoides Compounds |
|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons | ||||||
| 1 | α-pinene | 4.25 | 136.24 | C10H16 | 1.56 | - |
| 2 | (±)-β-pinene | 5.45 | 136.24 | C10H16 | 6.35 | - |
| 3 | D-limonene | 6.87 | 136.24 | C10H16 | 2.09 | - |
| 4 | γ-terpinene | 7.81 | 136.24 | C10H16 | 1.13 | - |
| 5 | p-cymene | 6.79 | 134.22 | C10H14 | 1.46 | - |
| 6 | - | 14.59 | 1.29 | p-cymene | ||
| Oxygenated monoterpenes | ||||||
| 7 | linalool | 9.44 | 154.25 | C10H18O | 2.43 | - |
| 8 | (−)-trans-pinocarveol | 11.10 | 152.24 | C10H16O | 3.37 | - |
| 9 | pinocarvone | 12.01 | 150.22 | C10H14O | 2.59 | - |
| 10 | 4-terpineol | 12.69 | 154.25 | C10H18O | 2.35 | - |
| 11 | (−)-myrtenol | 13.45 | 152.23 | C10H16O | 3.72 | |
| 12 | (−)-citronellol | 14.66 | 156.27 | C10H20O | 3.01 | - |
| 13 | - | 14.55 | 1.01 | (−)-citronellol | ||
| 14 | - | 15.44 | 148 | C10H12O | 1.00 | benzaldehyde, 4-(1-methylethyl)- |
| 15 | cuminic aldehyde | 15.50 | 148.2 | C10H12O | 2.19 | - |
| 16 | bornyl acetate | 16.84 | 196.29 | C12H20O2 | 1.79 | - |
| 17 | - | 20.88 | 196.29 | C12H20O2 | 1.67 | geranyl acetate |
| Oxygenated diterpene | ||||||
| 18 | - | 48.83 | 308.51 | C20H34O2 | 1.24 | cis-abienol |
| Non-oxygenated sesquiterpenes | ||||||
| 19 | - | 20.61 | 204.36 | C15H24 | 1.51 | α-gurjunene |
| 20 | α-curcumene | 22.19 | 202.34 | C15H22 | 5.72 | - |
| 21 | - | 24.99 | 7.49 | α-curcumene | ||
| 22 | - | 25.13 | 204.36 | C15H24 | 1.22 | α-selinene |
| 23 | 22.17 | 204.36 | C15H24 | 1.30 | beta-caryophyllene | |
| 24 | - | 24.76 | 204.36 | C15H24 | 1.46 | germacrene D |
| Oxygenated sesquiterpenes | ||||||
| 25 | - | 27.93 | 220.36 | C15H24O | 1.02 | ledene oxide-(ii) |
| 26 | (−)-caryophyllene oxide | 28.02 | 220.36 | C15H24O | 1.35 | - |
| 27 | - | 28.89 | 2.49 | (−)-caryophyllene oxide | ||
| 28 | isoaromadendrene epoxide | 28.25 | 220.36 | C15H24O | 1.29 | - |
| 29 | citronellyl iso-valerate | 28.79 | 240.39 | C15H28O2 | 2.76 | - |
| 30 | - | 28.67 | 4.09 | citronellyl iso-valerate | ||
| 31 | (−)-spathulenol | 28.99 | 220.36 | C15H24O | 2.21 | - |
| 32 | - | 28.79 | 2.83 | (−)-spathulenol | ||
| 33 | viridiflorol | 29.57 | 222.37 | C15H26O | 5.62 | - |
| 34 | - | 29.45 | 8.15 | viridiflorol | ||
| 35 | ledol | 29.86 | 222.37 | C15H26O | 1.18 | - |
| 36 | - | 30.07 | 222.37 | C15H26O | 5.66 | cubenol |
| 37 | widdrol | 30.26 | 222.37 | C15H26O | 7.57 | - |
| 38 | neoclovenoxid-alcohol | 30.44 | 220.36 | C15H24O | 1.20 | - |
| 39 | guaiol | 30.92 | 222.37 | C15H26O | 2.40 | - |
| 40 | - | 30.80 | 1.31 | guaiol | ||
| 41 | 31.35 | 222.37 | C15H26O | 4.63 | tau.-cadinol | |
| 42 | γ-eudesmol or eudesm-4-en-11-ol | 32.07 | 222.37 | C15H26O | 14.66 | - |
| 43 | - | 31.88 | 8.26 | γ-eudesmol or eudesm-4-en-11-ol | ||
| 44 | - | 32.03 | 218.34 | C15H22O | 1.09 | cis-nuciferol |
| 45 | - | 32.32 | 216.32 | C15H20O | 2.44 | ar-turmerone |
| 46 | - | 32.63 | 224.39 | C15H28O | 2.87 | 6,7-dihydro-2-cis-farnesol |
| 47 | - | 33.15 | 220.36 | C15H24O | 0.99 | aromadendrene oxide-(1) |
| 48 | hexahydrofarnesyl acetone | 38.34 | 268.49 | C18H36O | 2.39 | - |
| 49 | - | 38.32 | 2.00 | hexahydrofarnesyl acetone | ||
| 50 | - | 46.40 | 222.37 | C15H26O | 3.66 | (7a-isopropenyl-4,5-dimethyloctahydroinden-4-yl) methanol |
| Hydrocarbons | ||||||
| 51 | (1R)-(+)-nopinone or .beta-pinone | 11.19 | 138.21 | C9H14O | 1.31 | - |
| 52 | - | 15.31 | 196.29 | C12H20O2 | 2.20 | linalyl acetate |
| 53 | 2,4-pentadiynylbenzene | 17.38 | 140.19 | C11H8 | 2.37 | - |
| 54 | naphthalene, 2-ethenyl | 26.27 | 154.21 | C12H10 | 5.24 | - |
| 55 | - | 26.30 | 8.83 | naphthalene, 2-ethenyl- | ||
| 56 | 1,1′-biphenyl | 26.98 | 154.21 | C12H10 | 6.59 | - |
| 57 | - | 26.49 | 11.77 | 1,1′-biphenyl | ||
| 58 | oleamide | 54.57 | 281.48 | C18H35NO | 2.10 | - |
| 59 | - | 39.16 | 278.35 | C16H22O4 | 2.13 | diisobutyl phthalate |
| 60 | 46.40 | 191 | C10H9NOS | 4.39 | 2-(2-cyanoethylsulfanyl)benzaldehyde | |
| Ʃ = 100.0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sherbeny, G.A.; Dakhil, M.A.; Eid, E.M.; Abdelaal, M. Structural and Chemical Adaptations of Artemisia monosperma Delile and Limbarda crithmoides (L.) Dumort. in Response to Arid Coastal Environments along the Mediterranean Coast of Egypt. Plants 2021, 10, 481. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030481
El-Sherbeny GA, Dakhil MA, Eid EM, Abdelaal M. Structural and Chemical Adaptations of Artemisia monosperma Delile and Limbarda crithmoides (L.) Dumort. in Response to Arid Coastal Environments along the Mediterranean Coast of Egypt. Plants. 2021; 10(3):481. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030481
Chicago/Turabian StyleEl-Sherbeny, Ghada A., Mohammed A. Dakhil, Ebrahem M. Eid, and Mohamed Abdelaal. 2021. "Structural and Chemical Adaptations of Artemisia monosperma Delile and Limbarda crithmoides (L.) Dumort. in Response to Arid Coastal Environments along the Mediterranean Coast of Egypt" Plants 10, no. 3: 481. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030481