Decontamination and Germination of Buckwheat Grains upon Treatment with Oxygen Plasma Glow and Afterglow
Abstract
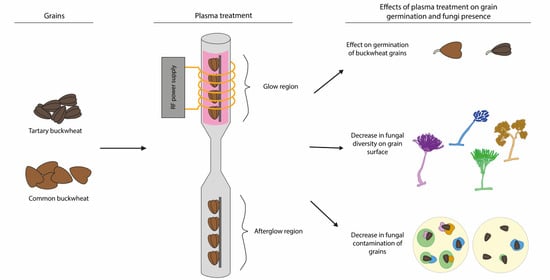
:1. Introduction
2. Results & Discussion
2.1. Plasma Characterisation
2.2. Efficacy of Decontamination
2.3. Effect of CP Treatment on Fungal Diversity
2.4. Effect of CP Treatment on Grain Germination
3. Materials and Methods
3.1. Buckwheat Grains Origin and Their Morphological Characteristics
3.2. Cold Plasma Treatment
3.3. Decontamination Test
3.3.1. Cultivation of Seed-Borne Fungi
3.3.2. Degree of Colonisation and Morphological Identification of Fungi
3.4. Germination Tests and Indices
3.5. Grain Characteristics
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO United Nations. How to Feed the World in 2050 Executive; FAO: Rome, Italy, 2009. [Google Scholar]
- FAO United Nations. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; ISBN 9789251095515. [Google Scholar]
- Committee on Microbiological Criteria for Foods. Microbiological safety evaluations and recommendations on sprouted seeds. Int. J. Food Microbiol. 1999, 52, 123–153. [Google Scholar] [CrossRef]
- Frisvald, J.C.; Samson, R.A. Filamentous Fungi in Foods and Feeds: Ecology, Spoilage, and Mycotoxin Production. In Handbook of Applied Mycology: Volume 3: Foods and Feeds; Arora, D.K., Mukerji, K.G., Marth, E.H., Eds.; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Halloin, J.M. Deterioration Resistance Mechanisms in Seeds. Phytopathology 1983, 73, 335–339. [Google Scholar] [CrossRef]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef]
- Miller, J.D. Fungi and mycotoxins in grains: Implication for stored roducts research. J. Stored Prod. Res. 1995, 31, 1–16. [Google Scholar] [CrossRef]
- Mancini, V.; Romanazzi, G. Seed treatments to control seedborne fungal pathogens of vegetable crops. Pest Manag. Sci. 2014, 70, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef] [Green Version]
- Scholtz, V.; Jirešová, J.; Šerá, B.; Julák, J. A review of microbial decontamination of cereals by non-thermal plasma. Foods 2021, 10, 2927. [Google Scholar] [CrossRef] [PubMed]
- Mravlje, J.; Regvar, M.; Vogel-Mikuš, K. Development of cold plasma technologies for surface decontamination of seed fungal pathogens: Present status and perspectives. J. Fungi 2021, 7, 650. [Google Scholar] [CrossRef]
- Randeniya, L.K.; De Groot, G.J.J.B. Non-Thermal Plasma Treatment of Agricultural Seeds for Stimulation of Germination, Removal of Surface Contamination and Other Benefits: A Review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of nonthermal plasma on morphology, genetics and physiology of seeds: A review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasmaactivated. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma agriculture: Review from the perspective of the plant and its ecosystem. Plasma Process. Polym. 2021, 18, e2000162. [Google Scholar] [CrossRef]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2018, 15, e1700174. [Google Scholar] [CrossRef]
- Waskow, A.; Butscher, D.; Oberbossel, G.; Klöti, D.; Rudolf von Rohr, P.; Büttner-Mainik, A.; Drissner, D.; Schuppler, M. Low-energy electron beam has severe impact on seedling development compared to cold atmospheric pressure plasma. Sci. Rep. 2021, 11, 16373. [Google Scholar] [CrossRef] [PubMed]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441–454. [Google Scholar] [CrossRef] [Green Version]
- Popović, D.; Mozetič, M.; Vesel, A.; Primc, G.; Zaplotnik, R. Review on vacuum ultraviolet generation in low-pressure plasmas. Plasma Process. Polym. 2021, 18, e2100061. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B Atom. Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Laroussi, M. Low temperature plasma-based sterilization: Overview and state-of-the-art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Fabjan, N. Nutritional comparison of tartary buckwheat with common buckwheat and minor cereals. Zb. Bioteh. Fak. Univ. Ljublj. Kmet 2003, 81, 349–355. [Google Scholar]
- Bonafaccia, G.; Gambelli, L.; Fabjan, N.; Kreft, I. Trace elements in flour and bran from common and tartary buckwheat. Food Chem. 2003, 83, 1–5. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and Tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Skerritt, J.H. Molecular Comparison of Alcohol-Soluble Wheat and Buchwheat Proteins. Cereal Chem. 1986, 63, 365–369. [Google Scholar]
- Kreft, I.; Zhou, M.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding buckwheat for nutritional quality. Breed. Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Popović, V.; Sikora, V.; Berenji, J.; Filipović, V.; Dolijanović, Ž.; Ikanović, J.; Dončić, D. Analysis of buckwheat production in the world and Serbia. Ekon. Poljopr. 2014, 61, 53–62. [Google Scholar] [CrossRef]
- Milevoj, L. Buckwheat diseases. In Fagopyrum (Buckwheat Newsletter); Kreft, I., Ed.; Biotehniška Fakulteta: Ljubljana, Slovenia, 1989; Volume 9, pp. 31–40. [Google Scholar]
- Šerá, B.; Gajdová, I.; Černák, M.; Gavril, B.; Hnatiuc, E.; Kováčik, D.; Kříha, V.; Sláma, J.; Šerý, M.; Špatenka, P. How various plasma sources may affect seed germination and growth. In Proceedings of the 13th International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Brasov, Romania, 24–26 May 2012; pp. 1365–1370. [Google Scholar] [CrossRef]
- Ivankov, A.; Naučienė, Z.; Degutytė-Fomins, L.; Žūkienė, R.; Januškaitienė, I.; Malakauskienė, A.; Jakštas, V.; Ivanauskas, L.; Romanovskaja, D.; Šlepetienė, A.; et al. Changes in agricultural performance of common buckwheat induced by seed treatment with cold plasma and electromagnetic field. Appl. Sci. 2021, 11, 4391. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Starič, P.; Mozetič, M.; Vogel-Mikuš, K. Cold plasma affects germination and fungal community structure of buckwheat seeds. Plants 2021, 10, 851. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Z.; Ma, T. The Effects of Plasma-Activated Water Treatment on the Growth of Tartary Buckwheat Sprouts. Front. Nutr. 2022, 9, 849615. [Google Scholar] [CrossRef]
- Pongrac, P.; Vogel-Mikuš, K.; Regvar, M.; Vavpetič, P.; Pelicon, P.; Kreft, I. Improved lateral discrimination in screening the elemental composition of buckwheat grain by micro-PIXE. J. Agric. Food Chem. 2011, 59, 1275–1280. [Google Scholar] [CrossRef]
- Basaran, P.; Ozcan, M. Occurrence of aflatoxins in various nuts commercialized in Turkey. J. Food Saf. 2009, 29, 95–105. [Google Scholar] [CrossRef]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.N.; Ayan, H.; Friedman, G. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Bol’shakov, A.A.; Cruden, B.A.; Mogul, R.; Rao, M.V.V.S.; Sharma, S.P.; Khare, B.; Meyyappan, M. Radio-frequency oxygen plasma as a sterilization source. AIAA J. 2004, 42, 823–832. [Google Scholar] [CrossRef]
- Cvelbar, U.; Vujoševič, D.; Vratnica, Z.; Mozetič, M. The influence of substrate material on bacteria sterilization in an oxygen plasma glow discharge. J. Phys. D Appl. Phys. 2006, 39, 3487–3493. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Dasan, B.G.; Mutlu, M.; Boyaci, I.H. Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor. Int. J. Food Microbiol. 2016, 216, 50–59. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Samson, R.A. (Eds.) Food Mycology: A Multifaced Approach to Fungi and Food; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kovačec, E.; Likar, M.; Regvar, M. Temporal changes in fungal communities from buckwheat seeds and their effects on seed germination and seedling secondary metabolism. Fungal Biol. 2016, 120, 666–678. [Google Scholar] [CrossRef]
- Mills, J.T.; Wallace, H.A.H. Microflora of buckwheat seed, changes in storage and effect of seed treatments on seedling emergence. Can. Plant Dis. Surv. 1971, 51, 154–158. [Google Scholar]
- Singh, P.N.; Sindhu, I.R.; Singhal, G. Fungi recorded from seeds and seedlings of Fagopyrum esculentum. J. Indian Bot. Soc. 1984, 63, 236–243. [Google Scholar]
- Kalinova, J.; Voženilkova, B.; Moudry, J. Occurrence of Fusarium spp and bacteria on surface of buckwheat achenes (Fagopyrum esculentum Moench). In Proceedings of the 9th International Symposium on Buckwheat, Prague, Czech Republic, 18–22 August 2004; pp. 491–493. [Google Scholar]
- Montie, T.C.; Kelly-Wintenberg, K.; Reece Roth, J. An overview of research using the one atmosphere uniform glow discharge plasma (OAUGDP) for sterilization of surfaces and materials. IEEE Trans. Plasma Sci. 2000, 28, 41–50. [Google Scholar] [CrossRef]
- Kopacki, M.; Pawlat, J.; Terebun, P.; Kwiatkowski, M.; Starek, A.; Kiczorowski, P. Efficacy of non-thermal plasma fumigation to control fungi occurring on onion seeds. In Proceedings of the 2017 International Conference on Electromagnetic Devices and Processes in Environment Protection with Seminar Applications of Superconductors (ELMECO & AoS), Naleczow, Poland, 3–6 December 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Šimek, M.; Morano, M.; De Miccolis Angelini, R.M.; Minafra, A.; Trotti, P.; Ambrico, M.; Prukner, V.; Faretra, F. Reduction of microbial contamination and improvement of germination of sweet basil (Ocimum basilicum L.) seeds via surface dielectric barrier discharge. J. Phys. D Appl. Phys. 2017, 50, 305401. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on Maize Seeds: Enhancement of Seedlings Growth and Surface Microorganisms Inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risks. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Fávaro, L.C.L.; de Sebastianes, F.L.S.; Araújo, W.L. Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS ONE 2012, 7, e36826. [Google Scholar] [CrossRef]
- Fávaro, D.L.C.L.; de Melo, F.L.; Aguilar-Vildoso, C.I.; Araújo, W.L. Polyphasic analysis of intraspecific diversity in Epicoccum nigrum warrants reclassification into separate species. PLoS ONE 2011, 6, e14828. [Google Scholar] [CrossRef] [Green Version]
- Da Costa Silveira, A.A.; Araújo, L.G.; de Fillipi, M.C.C.; Sibov, S.T. Isolation, identification and characterization of endophytic fungi of Bambusa oldhamii Munro applied as antagonists to Pyricularia oryzae. Rev. Ceres 2020, 67, 296–305. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Cai, L.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef] [Green Version]
- Hamayun, M.; Afzal Khan, S.; Ahmad, N.; Tang, D.S.; Kang, S.M.; Na, C.I.; Sohn, E.Y.; Hwang, Y.H.; Shin, D.H.; Lee, B.H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Răut, I.; Călin, M.; Capră, L.; Gurban, A.-M.; Doni, M.; Radu, N.; Jecu, L. Cladosporium sp. Isolate as Fungal Plant Growth Promoting Agent. Agronomy 2021, 11, 392. [Google Scholar] [CrossRef]
- AlMatar, M.; Makky, E.A. Cladosporium cladosporioides from the perspectives of medical and biotechnological approaches. 3 Biotech 2016, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Christensen, C.M. Deterioration of Stored Grains by Fungi. Bot. Rev. 1957, 23, 108–134. [Google Scholar] [CrossRef]
- Essemine, J.; Ammar, S.; Bouzid, S. Impact of Heat Stress on Germination and Growth in Higher Plants: Physiological, Biochemical and Molecular Repercussions and Mechanisms of Defence. J. Biol. Sci. 2010, 10, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Starič, P.; Mlakar, S.G.; Junkar, I. Response of Two Different Wheat Varieties to Glow and Afterglow Oxygen Plasma. Plants 2021, 10, 1728. [Google Scholar] [CrossRef] [PubMed]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Tang, J.; Sokhansanj, S.; Yannacopoulos, S.; Kasap, S.O. Specific heat capacity of lentil seeds by differential scanning calorimetry. Trans. Am. Soc. Agric. Eng. 1991, 34, 517–522. [Google Scholar] [CrossRef]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi, Morphologies of Cultured Fungi and Key to Species, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 0849311187. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: Boston, MA, USA, 2013; ISBN 9788578110796. [Google Scholar]
- Ellis, R.A.; Roberts, E.H. The quantification of aging and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Ahmad, N.; Hafeez, K. Thermal hardening: A new seed vigor enhancement tool in rice. J. Integr. Plant Biol. 2005, 47, 187–193. [Google Scholar] [CrossRef]








| Treatment | Common Buckwheat | Tartary Buckwheat |
|---|---|---|
| Control | 1.886 | 2.122 |
| Vacuum Control | 1.852 | 1.940 |
| Glow 30 s | 1.383 | 1.273 |
| Glow 60 s | 1.496 | 1.475 |
| Afterglow 60 s | 1.547 | 1.688 |
| Afterglow 120 s | 1.675 | 1.636 |
| Afterglow 180 s | 1.630 | 1.448 |
| Sterilised Control | 0 | 0 |
| CB | TB | |||
|---|---|---|---|---|
| Treatment | MGT | T50 | MGT | T50 |
| Control | 4.87 a | 1.01 a | 5.38 a | 1.65 a |
| Vacuum C. | 4.84 a | 0.96 a | 5.32 a | 1.60 a |
| Glow 30 s | n.d. | n.d. | n.d. | n.d. |
| Glow 60 s | n.d. | n.d. | n.d. | n.d. |
| AGlow 60 s | 6.84 c | 4.05 b | 6.18 b | 2.67 b |
| AGlow 120 s | 6.59 b | 4.12 b | 6.27 b | 2.77 bc |
| AGlow 180 s | 6.75 bc | 3.88 b | 6.33 b | 2.92 c |
| Parameter | Common Buckwheat | Tartary Buckwheat |
|---|---|---|
| Weight (mg) | 25.9 ± 0.9 a | 16.3 ± 0.8 b |
| Length (mm) | 5.33 ± 0.1 a | 4.96 ± 0.1 b |
| Width (mm) | 3.87 ± 0.0 a | 2.75 ± 0.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mravlje, J.; Regvar, M.; Starič, P.; Zaplotnik, R.; Mozetič, M.; Vogel-Mikuš, K. Decontamination and Germination of Buckwheat Grains upon Treatment with Oxygen Plasma Glow and Afterglow. Plants 2022, 11, 1366. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11101366
Mravlje J, Regvar M, Starič P, Zaplotnik R, Mozetič M, Vogel-Mikuš K. Decontamination and Germination of Buckwheat Grains upon Treatment with Oxygen Plasma Glow and Afterglow. Plants. 2022; 11(10):1366. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11101366
Chicago/Turabian StyleMravlje, Jure, Marjana Regvar, Pia Starič, Rok Zaplotnik, Miran Mozetič, and Katarina Vogel-Mikuš. 2022. "Decontamination and Germination of Buckwheat Grains upon Treatment with Oxygen Plasma Glow and Afterglow" Plants 11, no. 10: 1366. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11101366