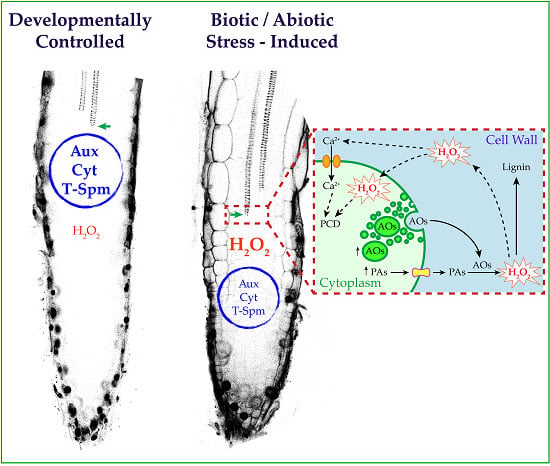
Cell Wall Amine Oxidases: New Players in Root Xylem Differentiation under Stress Conditions
Abstract
:1. Introduction
2. Polyamines as Signaling Compounds and/or Hydrogen Peroxide Sources
2.1. Polyamines in Plants
2.2. Polyamine Signaling in Xylem Development
3. Terminal Polyamine Oxidation in the Cell Wall Is Triggered at Specific Developmental Stages or under Stress Conditions
3.1. Polyamines Are Oxidized by Copper and FAD-Dependent Amine Oxidases

3.2. Features and Roles of Polyamine Oxidation in the Apoplast
4. Apoplastic Spermidine Oxidation Mediates Early Xylem Differentiation in the Maize Primary Root
4.1. ZmPAO Expression and Sub-Cellular Distribution Are Developmentally Regulated in Maize Primary Root
4.2. ZmPAO-Driven Oxidation of Spermidine in the Apoplast Mediates Early Xylem Differentiation in Maize Primary Root
5. Cell Wall PAO and CuAO Signal Early Xylem Differentiation in Roots of Tobacco Plants
5.1. Overexpression of ZmPAO in Tobacco Plants Induces Early Differentiation of Root Vascular Tissues
5.2. CuAO Mediates Early Xylem Differentiation in Transgenic Tobacco Plants with Constitutively-Activated Defense Responses
6. Hydrogen Peroxide Produced by the Apoplastic Copper Amine Oxidase 1 (AtAO1) Signals the Methyl-Jasmonate-Mediated Protoxylem Differentiation in Arabidopsis Roots
7. Conclusions and Future Perspective

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verbelen, J.P.; de Cnodder, T.; Le, J.; Vissenberg, K.; Baluska, F. The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities: Meristematic zone, transition zone, fast elongation zone and growth terminating zone. Plant Signal. Behav. 2006, 1, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, F.; Suzuki, M.; Suzuki, H. Examination of morphological changes in the first formed protoxylem in Arabidopsis seedlings. J. Plant Res. 2002, 115, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Rost, T.L.; Baum, S. On the correlation of primary root length, meristem size and protoxylem tracheary element position in pea seedlings. Am. J. Bot. 1988, 75, 414–424. [Google Scholar] [CrossRef]
- Perilli, S.; di Mambro, R.; Sabatini, S. Growth and development of the root apical meristem. Curr. Opin. Plant Biol. 2012, 15, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis root development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Mähönen, A.P.; ten Tusscher, K.; Siligato, R.; Smetana, O.; Díaz-Triviño, S.; Salojärvi, J.; Wachsman, G.; Prasad, K.; Heidstra, R.; Scheres, B. PLETHORA gradient formation mechanism separates auxin responses. Nature 2014, 515, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bishopp, A.; Help, H.; El-Showk, S.; Weijers, D.; Scheres, B.; Friml, J.; Benková, E.; Mähönen, A.P.; Helariutta, Y. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr. Biol. 2011, 21, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Mähönen, A.P.; Bishopp, A.; Higuchi, M.; Nieminen, K.M.; Kinoshita, K.; Törmäkangas, K.; Ikeda, Y.; Oka, A.; Kakimoto, T.; Helariutta, Y. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science 2006, 311, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, J.; Zhai, Q.; Zhou, W.; Qi, L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.; Qi, J.; et al. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, H.; Busch, W.; Benfey, P.N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 2010, 143, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Mattoo, A.K. Differential and functional interactions emphasize the multiple roles of polyamines in plants. Plant Physiol. Biochem. 2010, 48, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, e175. [Google Scholar] [CrossRef] [PubMed]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Kakehi, J.; Takahashi, T. Thermospermine is not a minor polyamine in the plant kingdom. Plant Cell Physiol. 2012, 53, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Tavladoraki, P.; Cona, A.; Federico, R.; Tempera, G.; Viceconte, N.; Saccoccio, S.; Battaglia, V.; Toninello, A.; Agostinelli, E. Polyamine catabolism: Target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids 2012, 42, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Kakehi, J. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 2010, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Angelini, R.; Cona, A.; Federico, R.; Fincato, P.; Tavladoraki, P.; Tisi, A. Plant amine oxidases “on the move”: An update. Plant Physiol. Biochem. 2010, 48, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 2008, 20, 1708–1724. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Angelini, R.; Cona, A. Does polyamine catabolism influence root development and xylem differentiation under stress conditions? Plant Signal. Behav. 2011, 11, 1844–1847. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Cui, X.; Wang, Y.; Hu, Y.; Fu, Z.; Zhang, D.; Cheng, Z.; Li, J. BUD2, encoding an S-adenosylmethionine decarboxylase, is required for Arabidopsis growth and development. Cell Res. 2006, 16, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Ge, C.; Wang, R.; Wang, H.; Chen, W.; Fu, Z.; Jiang, X.; Li, J.; Wang, Y. The BUD2 mutation affects plant architecture through altering cytokinin and auxin responses in Arabidopsis. Cell Res. 2010, 20, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Clay, N.K.; Nelson, T. Arabidopsis thick vein mutation affects vein thickness and organ vascularization, and resides in a provascular cell-specific spermine synthase involved in vein definition and in polar auxin transport. Plant Physiol. 2005, 138, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, L.; Minguet, E.G.; Singh, S.K.; Pesquet, E.; Vera-Sirera, F.; Moreau-Courtois, C.L.; Carbonell, J.; Blázquez, M.A.; Tuominen, H. ACAULIS5 controls Arabidopsis xylem specification through the prevention of premature cell death. Development 2008, 135, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Vera-Sirera, F.; Minguet, E.G.; Singh, S.K.; Ljung, K.; Tuominen, H.; Blázquez, M.A.; Carbonell, J. Role of polyamines in plant vascular development. Plant Physiol. Biochem. 2010, 48, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Baima, S.; Forte, V.; Possenti, M.; Peñalosa, A.; Leoni, G.; Salvi, S.; Felici, B.; Ruberti, I.; Morelli, G. Negative feedback regulation of auxin signaling by ATHB8/ACL5-BUD2 transcription module. Mol. Plant 2014, 7, 1006–1025. [Google Scholar] [CrossRef] [PubMed]
- Donner, T.J.; Sherr, I.; Scarpella, E. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 2009, 136, 3235–3246. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Yoshimoto, K.; Kakehi, J.; Motose, H.; Niitsu, M.; Takahashi, T. Thermospermine modulates expression of auxin-related genes in Arabidopsis. Front. Plant Sci. 2014, 5, e94. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Hummel, I.; Sulmon, C.; Gouesbet, G.; Amrani, A.E.I. Involvement of polyamines in root development. Plant Cell Tissue Organ. Cult. 2004, 76, 1–10. [Google Scholar] [CrossRef]
- De Agazio, M.; Federico, R.; Angelini, R.; de Cesare, F.; Grego, S. Spermidine pretreatment or root tip removal in maize seedlings: Effects on K+ uptake and tissue modifications. J. Plant Physiol. 1992, 140, 741–746. [Google Scholar] [CrossRef]
- De Agazio, M.; Grego, S.; Ciofi-Luzzatto, A.; Rea, E.; Zaccaria, M.L.; Federico, R. Inhibition of maize primary root elongation by spermidine: Effect on cell shape and mitotic index. Plant Growth Regul. 1995, 14, 85–89. [Google Scholar] [CrossRef]
- Angelini, R.; Tisi, A.; Rea, G.; Chen, M.M.; Botta, M.; Federico, R.; Cona, A. Involvement of polyamine oxidase in wound healing. Plant Physiol. 2008, 146, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Tisi, A.; Ghuge, S.A.; Franchi, S.; de Lorenzo, G.; Angelini, R. Wound healing response and xylem differentiation in tobacco plants over-expressing a fungal endopolygalacturonase is mediated by copper amine oxidase activity. Plant Physiol. Biochem. 2014, 82, 54–65. [Google Scholar] [CrossRef] [PubMed]
- De Agazio, M.; Federico, R.; Grego, S. Involvement of polyamines in the inhibiting effect of injury caused by cutting on K(+) uptake through the plasma membrane. Planta 1989, 177, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Pietrangeli, P.; Morpurgo, L.; Mondovì, B.; di Paolo, M.L.; Rigo, A. Soluble copper amine oxidases from mammals. In Copper Amine Oxidases: Structure, Catalytic Mechanism and Role in Pathophysiology; Floris, G., Mondovì, B., Eds.; Taylor and Francis group, C.R.C. Press: Boca Raton, FL, USA, 2009; Volume 5, pp. 51–68. [Google Scholar]
- Boudart, G.; Jamet, E.; Rossignol, M.; Lafitte, C.; Borderies, G.; Jauneau, A.; Esquerré-Tugayé, M.T.; Pont-Lezica, R. Cell wall proteins in apoplastic fluids of Arabidopsis thaliana rosettes: Identification by mass spectrometry and bioinformatics. Proteomics 2005, 5, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.G.; McPherson, M.J. Developmental expression and biochemical analysis of the Arabidopsis atao1 gene encoding an H2O2-generating diamine oxidase. Plant J. 1998, 13, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Naconsie, M.; Kato, K.; Shoji, T.; Hashimoto, T. Molecular evolution of N-methylputrescine oxidase in tobacco. Plant Cell Physiol. 2014, 55, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reumann, S.; Quan, S.; Aung, K.; Yang, P.; Manandhar-Shrestha, K.; Holbrook, D.; Linka, N.; Switzenberg, R.; Wilkerson, C.G.; Weber, A.P.; et al. In-depth proteome analysis of Arabidopsis leaf peroxisomes combined with in vivo subcellular targeting verification indicates novel metabolic and regulatory functions of peroxisomes. Plant Physiol. 2009, 150, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Trobacher, C.P.; Cooke, A.R.; Meyers, A.J.; Hall, J.C.; Shelp, B.J. Apple fruit copper amine oxidase isoforms: Peroxisomal MdAO1 prefers diamines as substrates, whereas extracellular MdAO2 exclusively utilizes monoamines. Plant Cell Physiol. 2015, 56, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Marina, M.; Maiale, S.J.; Rossi, F.R.; Romero, M.F.; Rivas, E.I.; Gárriz, A.; Ruiz, O.A.; Pieckenstain, F.L. Apoplastic polyamine oxidation plays different roles in local responses of tobacco to infection by the necrotrophic fungus Sclerotinia sclerotiorum and the biotrophic bacterium Pseudomonas viridiflava. Plant Physiol. 2008, 147, 2164–2178. [Google Scholar] [CrossRef] [PubMed]
- Fincato, P.; Moschou, P.N.; Spedaletti, V.; Tavazza, R.; Angelini, R.; Federico, R.; Roubelakis-Angelakis, K.A.; Tavladoraki, P. Functional diversity inside the Arabidopsis polyamine oxidase gene family. J. Exp. Bot. 2011, 62, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Tavladoraki, P.; Rossi, M.N.; Saccuti, G.; Perez-Amador, M.A.; Polticelli, F.; Angelini, R.; Federico, R. Heterologous expression and biochemical characterization of a polyamine oxidase from Arabidopsis involved in polyamine back conversion. Plant Physiol. 2006, 141, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Kim, D.W.; Niitsu, M.; Berberich, T.; Kusano, T. Oryza sativa polyamine oxidase 1 back-converts tetraamines, spermine and thermospermine, to spermidine. Plant Cell Rep. 2014, 33, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Kim, D.W.; Watanabe, K.; Sasaki, A.; Niitsu, M.; Berberich, T.; Kusano, T.; Takahashi, Y. Constitutively and highly expressed Oryza sativa polyamine oxidases localize in peroxisomes and catalyze polyamine back conversion. Amino Acids 2012, 42, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Ahou, A.; Martignago, D.; Alabdallah, O.; Tavazza, R.; Stano, P.; Macone, A.; Pivato, M.; Masi, A.; Rambla, J.L.; Vera-Sirera, F.; et al. A plant spermine oxidase/dehydrogenase regulated by the proteasome and polyamines. J. Exp. Bot. 2014, 65, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Watanabe, K.; Murayama, C.; Izawa, S.; Niitsu, M.; Michael, A.J.; Berberich, T.; Kusano, T. Polyamine oxidase 5 regulates Arabidopsis growth through thermospermine oxidase activity. Plant Physiol. 2014, 165, 1575–1590. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Kim, D.W.; Niitsu, M.; Maeda, S.; Watanabe, M.; Kamio, Y.; Berberich, T.; Kusano, T. Polyamine oxidase 7 is a terminal catabolism-type enzyme in Oryza sativa and is specifically expressed in anthers. Plant Cell Physiol. 2014, 55, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Rea., G.; de Pinto, M.C.; Tavazza, R.; Biondi, S.; Gobbi, V.; Ferrante, P.; de Gara, L.; Federico, R.; Angelini, R.; Tavladoraki, P. Ectopic expression of maize polyamine oxidase and pea copper amine oxidase in the cell wall of tobacco plants. Plant Physiol. 2004, 134, 1414–1426. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.A.; Maiale, S.J.; Menéndez, A.B.; Ruiz, O.A. Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J. Exp. Bot. 2009, 60, 4249–4262. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Kamada, H.; Satoh, M.; Ohashi, Y. Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection. Plant Physiol. 1998, 118, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Yoda, H.; Fujimura, K.; Takahashi, H.; Munemura, I.; Uchimiya, H.; Sano, H. Polyamines as a common source of hydrogen peroxide in host- and nonhost hypersensitive response during pathogen infection. Plant Mol. Biol. 2009, 70, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Cenci, F.; Cervelli, M.; Federico, R.; Mariottini, P.; Moreno, S.; Angelini, R. Polyamine oxidase, a hydrogen peroxide-producing enzyme, is up-regulated by light and down-regulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol. 2003, 131, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Moreno, S.; Cenci, F.; Federico, R.; Angelini, R. Cellular redistribution of flavin-containing polyamine oxidase in differentiating root and mesocotyl of Zea mays L. seedlings. Planta 2005, 221, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. TRENDS Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Kärkönen, A.; Kuchitsu, K. Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 2015, 112, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Medda, R.; Bellelli, A.; Peč, P.; Federico, R.; Cona, A.; Floris, G. Copper Amine oxidases from plants. In Copper Amine Oxidases: Structure, Catalytic Mechanism and Role in Pathophysiology; Floris, G., Mondovì, B., Eds.; Taylor and Francis Group, C.R.C. Press: Boca Raton, FL, USA, 2009; Volume 4, pp. 39–50. [Google Scholar]
- Ghuge, S.A.; Carucci, A.; Rodrigues Pousada, R.A.; Tisi, A.; Franchi, S.; Tavladoraki, P.; Angelini, R.; Cona, A. The apoplastic copper AMINE OXIDASE1 mediates jasmonic acid-induced protoxylem differentiation in Arabidopsis roots. Plant Physiol. 2015, 168, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 5, e470. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, Y.; Takahashi, Y.; Berberich, T.; Miyazaki, A.; Matsumura, H.; Takahashi, H.; Terauchi, R.; Kusano, T. Spermine signaling plays a significant role in the defense response of Arabidopsis thaliana to cucumber mosaic virus. J. Plant Physiol. 2009, 166, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Yoda, H.; Hiro, Y.; Sano, H. Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol. 2006, 142, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Rea, G.; Metoui, O.; Infantino, A.; Federico, R.; Angelini, R. Copper amine oxidase expression in defense responses to wounding and Ascochyta rabiei invasion. Plant Physiol. 2002, 128, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Colville, L.; Beckett, R.P.; Minibayeva, F.V.; Havaux, M.; Kranner, I. A proposed interplay between peroxidase, amine oxidase and lipoxygenase in the wounding-induced oxidative burst in Pisum sativum seedlings. Phytochemistry 2015, 112, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Angelini, R.; Cona, A. Wound healing in plants: Cooperation of copper amine oxidase and flavin-containing polyamine oxidase. Plant Signal. Behav. 2008, 3, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Federico, R.; Moreno, S.; Lucretti, S.; Moschou, P.N.; Roubelakis-Angelakis, K.A.; Angelini, R.; Cona, A. Perturbation of polyamine catabolism can strongly affect root development and xylem differentiation. Plant Physiol. 2011, 157, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Cona., A.; Rea, G.; Botta, M.; Corelli, F.; Federico, R.; Angelini, R. Flavin-containing polyamine oxidase is a hydrogen peroxide source in the oxidative response to the protein phosphatase inhibitor cantharidin in Zea mays L. J. Exp. Bot. 2006, 57, 2277–2289. [Google Scholar] [CrossRef] [PubMed]
- Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Spatial and temporal distribution of polyamine levels and polyamine anabolism in different organs/tissues of the tobacco plant. Correlations with age, cell division/expansion, and differentiation. Plant Physiol. 2005, 138, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Galletti, R.; Pontiggia, D.; Manfredini, C.; Lionetti, V.; Bellincampi, D.; Cervone, F.; de Lorenzo, G. Transgenic expression of a fungal endo-polygalacturonase increases plant resistance to pathogens and reduces auxin sensitivity. Plant Physiol. 2008, 146, 669–681. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghuge, S.A.; Tisi, A.; Carucci, A.; Rodrigues-Pousada, R.A.; Franchi, S.; Tavladoraki, P.; Angelini, R.; Cona, A. Cell Wall Amine Oxidases: New Players in Root Xylem Differentiation under Stress Conditions. Plants 2015, 4, 489-504. https://0-doi-org.brum.beds.ac.uk/10.3390/plants4030489
Ghuge SA, Tisi A, Carucci A, Rodrigues-Pousada RA, Franchi S, Tavladoraki P, Angelini R, Cona A. Cell Wall Amine Oxidases: New Players in Root Xylem Differentiation under Stress Conditions. Plants. 2015; 4(3):489-504. https://0-doi-org.brum.beds.ac.uk/10.3390/plants4030489
Chicago/Turabian StyleGhuge, Sandip A., Alessandra Tisi, Andrea Carucci, Renato A. Rodrigues-Pousada, Stefano Franchi, Paraskevi Tavladoraki, Riccardo Angelini, and Alessandra Cona. 2015. "Cell Wall Amine Oxidases: New Players in Root Xylem Differentiation under Stress Conditions" Plants 4, no. 3: 489-504. https://0-doi-org.brum.beds.ac.uk/10.3390/plants4030489