Embossed Membranes with Vascular Patterns Guide Vascularization in a 3D Tissue Model
Abstract
:1. Introduction
2. Materials and Methods
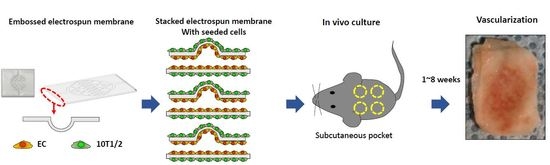
2.1. Embossed Electrospun Membrane Generation (Vacuum Forming)
2.2. Preparation of Cell Sheet for Cell Culture
2.3. Cell Culture
2.4. In Vivo Experiments
2.5. Harvesting and Fixation of Implanted Tissue Sheets
2.6. Immunofluorescence Staining
2.7. Quantification of Immunofluorescence Staining
2.8. Statistical Analysis
3. Results
3.1. Forming of Embossed PCL Electrospun Membrane and Cell Culture on Embossed Membrane
3.2. Gross Observations
3.3. Analysis of Vascularization
3.4. Vascular Maturation and Anastomosis of Vascularization
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 300–311. [Google Scholar]
- Muehleder, S.; Ovsianikov, A.; Zipperle, J.; Redl, H.; Holnthoner, W. Connections matter: Channeled hydrogels to improve vascularization. Front. Bioeng. Biotechnol. 2014, 2, 52. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005; p. 396. [Google Scholar]
- Alamein, M.A.; Liu, Q.; Stephens, S.; Skabo, S.; Warnke, F.; Bourke, R.; Heiner, P.; Warnke, P.H. Nanospiderwebs: Artificial 3d extracellular matrix from nanofibers by novel clinical grade electrospinning for stem cell delivery. Adv. Healthc. Mater. 2013, 2, 702–717. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Hong, S.; Jung, B.Y.; Hwang, C. Multilayered engineered tissue sheets for vascularized tissue regeneration. Tissue Eng. Regen. Med. 2017, 14, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.A.; Landázuri, N.; Thulé, P.M.; Taylor, W.R.; García, A.J. Bioartificial matrices for therapeutic vascularization. Proc. Natl. Acad. Sci. USA 2010, 107, 3323–3328. [Google Scholar] [CrossRef]
- Plovie, B.; Yang, Y.; Guillaume, J.; Dunphy, S.; Dhaenens, K.; Van Put, S.; Vandecasteele, B.; Vervust, T.; Bossuyt, F.; Vanfleteren, J. Arbitrarily shaped 2.5d circuits using stretchable interconnects embedded in thermoplastic polymers. Adv. Eng. Mater. 2017, 19, 1700032. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Effects of shear stress on endothelial cells: Go with the flow. Acta Physiol. 2017, 219, 382–408. [Google Scholar] [CrossRef]
- Davies, P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. 2009, 6, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, S.H.; Porter, B.D.; Garcia, A.J.; Guldberg, R.E. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003, 9, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Grayson, W.L.; Bhumiratana, S.; Cannizzaro, C.; Chao, P.H.; Lennon, D.P.; Caplan, A.I.; Vunjak-Novakovic, G. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng. Part A 2008, 14, 1809–1820. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: Dynamic cell seeding and construct development. Biotechnol. Bioeng. 2005, 91, 482–493. [Google Scholar] [CrossRef]
- Radisic, M.; Yang, L.; Boublik, J.; Cohen, R.J.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H507–H516. [Google Scholar] [CrossRef] [Green Version]
- Takebe, T.; Zhang, R.R.; Koike, H.; Kimura, M.; Yoshizawa, E.; Enomura, M.; Koike, N.; Sekine, K.; Taniguchi, H. Generation of a vascularized and functional human liver from an ipsc-derived organ bud transplant. Nat. Protoc. 2014, 9, 396–409. [Google Scholar] [CrossRef]
- Hoffmann, A.; Bredno, J.; Wendland, M.; Derugin, N.; Ohara, P.; Wintermark, M. High and low molecular weight fluorescein isothiocyanate (fitc)-dextrans to assess blood-brain barrier disruption: Technical considerations. Transl. Stroke Res. 2011, 2, 106–111. [Google Scholar] [CrossRef]
- Baras, A.S.; Solomon, A.; Davidson, R.; Moskaluk, C.A. Loss of vopp1 overexpression in squamous carcinoma cells induces apoptosis through oxidative cellular injury. Lab. Investig. 2011, 91, 1170–1180. [Google Scholar] [CrossRef]
- Barbachano, A.; Ordonez-Moran, P.; Garcia, J.M.; Sanchez, A.; Pereira, F.; Larriba, M.J.; Martinez, N.; Hernandez, J.; Landolfi, S.; Bonilla, F.; et al. Sprouty-2 and e-cadherin regulate reciprocally and dictate colon cancer cell tumourigenicity. Oncogene 2010, 29, 4800–4813. [Google Scholar] [CrossRef]
- Hay, J.J.; Rodrigo-Navarro, A.; Hassi, K.; Moulisova, V.; Dalby, M.J.; Salmeron-Sanchez, M. Living biointerfaces based on non-pathogenic bacteria support stem cell differentiation. Sci. Rep. 2016, 6, 21809. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Yun, J.H.; Kim, E.-S.; Kim, J.S.; Tchah, H.; Hwang, C. Human conjunctival epithelial sheets grown on poly(lactic-co-glycolic) acid membranes and cocultured with human tenon’s fibroblasts for corneal repairengineered human conjunctival epithelial sheet. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Abarrategi, A.; Mian, S.A.; Passaro, D.; Rouault-Pierre, K.; Grey, W.; Bonnet, D. Modeling the human bone marrow niche in mice: From host bone marrow engraftment to bioengineering approaches. J. Exp. Med. 2018, 215, 729–743. [Google Scholar] [CrossRef] [Green Version]
- Brudno, Y.; Ennett-Shepard, A.B.; Chen, R.R.; Aizenberg, M.; Mooney, D.J. Enhancing microvascular formation and vessel maturation through temporal control over multiple pro-angiogenic and pro-maturation factors. Biomaterials 2013, 34, 9201–9209. [Google Scholar] [CrossRef] [PubMed]
- Collen, D.; Conway, E.M.; Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [Green Version]
- Jeon, J.S.; Bersini, S.; Whisler, J.A.; Chen, M.B.; Dubini, G.; Charest, J.L.; Moretti, M.; Kamm, R.D. Generation of 3d functional microvascular networks with human mesenchymal stem cells in microfluidic systems. Integr. Biol. 2014, 6, 555–563. [Google Scholar] [CrossRef]
- Koblizek, T.I.; Weiss, C.; Yancopoulos, G.D.; Deutsch, U.; Risau, W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Current Biology 1998, 8, 529–532. [Google Scholar] [CrossRef] [Green Version]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncology 2005, 7, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; da Silva, M.L.A.; Faria, S.; Marques, A.P.; Reis, R.L.; Neves, N.M. The influence of patterned nanofiber meshes on human mesenchymal stem cell osteogenesis. Macromol. Biosci. 2011, 11, 978–987. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Wang, G.X.; Chen, L.; Li, H.; Yin, T.Y.; Wang, B.C.; Lee, J.C.M.; Yu, Q.S. Electrospun nanofiber meshes with tailored architectures and patterns as potential tissue-engineering scaffolds. Biofabrication 2009, 1, 015001. [Google Scholar] [CrossRef]
- Zhang, D.M.; Chang, J. Patterning of electrospun fibers using electroconductive templates. Adv. Mater. 2007, 19, 3664. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Xu, H.; Chang, J. Electrospun membranes: Control of the structure and structure related applications in tissue regeneration and drug delivery. J. Mater. Chem. B 2014, 2, 5492–5510. [Google Scholar] [CrossRef]
- Freiman, A.; Shandalov, Y.; Rosenfeld, D.; Shor, E.; Ben-David, D.; Meretzki, S.; Levenberg, S.; Egozi, D. Engineering vascularized flaps using adipose-derived microvascular endothelial cells and mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2018, 12, e130–e141. [Google Scholar] [CrossRef]
- Yap, K.K.; Yeoh, G.C.; Morrison, W.A.; Mitchell, G.M. The vascularised chamber as an in vivo bioreactor. Trends Biotechnol. 2018, 36, 1011–1024. [Google Scholar] [CrossRef]
- Huang, R.L.; Liu, K.; Li, Q. Bone regeneration following the in vivo bioreactor principle: Is in vitro manipulation of exogenous elements still needed? Regen. Med. 2016, 11, 475–481. [Google Scholar] [CrossRef]
- Akar, B.; Tatara, A.M.; Sutradhar, A.; Hsiao, H.-Y.; Miller, M.; Cheng, M.-H.; Mikos, A.G.; Brey, E.M. Large animal models of an in vivo bioreactor for engineering vascularized bone. Tissue Eng. Part B Rev. 2018, 24, 317–325. [Google Scholar] [CrossRef]
- Koike, N.; Fukumura, D.; Gralla, O.; Au, P.; Schechner, J.S.; Jain, R.K. Creation of long-lasting blood vessels. Nature 2004, 428, 138. [Google Scholar] [CrossRef]
- Cheng, G.; Liao, S.; Kit Wong, H.; Lacorre, D.A.; di Tomaso, E.; Au, P.; Fukumura, D.; Jain, R.K.; Munn, L.L. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood 2011, 118, 4740–4749. [Google Scholar] [CrossRef] [Green Version]
- Qin, D.; Trenkwalder, T.; Lee, S.; Chillo, O.; Deindl, E.; Kupatt, C.; Hinkel, R. Early vessel destabilization mediated by angiopoietin-2 and subsequent vessel maturation via angiopoietin-1 induce functional neovasculature after ischemia. PLoS ONE 2013, 8, e61831. [Google Scholar] [CrossRef]
- Darland, D.C.; D’Amore, P.A. Tgf beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10t1/2 and endothelial cells. Angiogenesis 2001, 4, 11–20. [Google Scholar] [CrossRef]
- Moon, J.J.; Saik, J.E.; Poché, R.A.; Leslie-Barbick, J.E.; Lee, S.H.; Smith, A.A.; Dickinson, M.E.; West, J.L. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 2010, 31, 3840–3847. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.E.; Gou, M.; Xu, Y.; et al. Direct 3d bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Rohovsky, S.A.; D’Amore, P.A. Pdgf, tgf-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10t1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol. 1998, 141, 805–814. [Google Scholar] [CrossRef]
- Kang, Y.; Chang, J. Channels in a porous scaffold: A new player for vascularization. Future Med. 2018, 13, 705–715. [Google Scholar] [CrossRef]
- Huang, G.; Wang, S.; He, X.; Zhang, X.; Lu, T.J.; Xu, F. Helical spring template fabrication of cell-laden microfluidic hydrogels for tissue engineering. Biotechnol. Bioeng. 2013, 110, 980–989. [Google Scholar] [CrossRef]
- Chiu, L.L.; Montgomery, M.; Liang, Y.; Liu, H.; Radisic, M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc. Natl. Acad. Sci. USA 2012, 109, E3414–E3423. [Google Scholar] [CrossRef] [Green Version]
- Garvin, K.A.; Dalecki, D.; Hocking, D.C. Vascularization of three-dimensional collagen hydrogels using ultrasound standing wave fields. Ultrasound Med. Boil. 2011, 37, 1853–1864. [Google Scholar] [CrossRef]
- Golden, A.P.; Tien, J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip 2007, 7, 720–725. [Google Scholar] [CrossRef]









| Group Name | Number of Embossed Membranes in Laminated Assembly | Number of Assembled Membranes | Number of Stacked Structures |
|---|---|---|---|
| 2× flat | 0 | 2 | 16 |
| 2× embossed | 1 | 2 | 16 |
| 6× flat | 0 | 6 | 16 |
| 6× embossed | 3 | 6 | 16 |
| Name | Type | Dilution | Company, No. | Annotation |
|---|---|---|---|---|
| Anti-VE-cadherin | poly | 1:100 | Abcam Ab33168 | Endothelial cell marker |
| Anti-CD31 | mono | 1:100 | Thermo MA3100 | Endothelial cell marker |
| Anti-Ang-1 | poly | 1:100 | Abcam Ab8451 | Vascularization |
| Anti-VEGF | mono | 1:100 | Abcam Ab1316 | Vascularization |
| Anti-Human mitochondria | mono | 1:100 | Sigma MAB1273 | Human cell marker |
| Anti-α smooth muscle actin | poly | 1:100 | Abcam Ab5694 | Vessel maturation |
| Phalloidin | - | 1:40 | Thermo R415 | F-actin |
| 4’,6-Diamidino-2-Phenylindole, Dihydrochloride | 1:100 | Thermo D1306 | Cell nuclear staining | |
| Alexa Fluor 488 goat anti-Mouse | poly | 1:100 | Thermo A11001 | 2nd antibody |
| Alexa Fluor 488 goat anti-Rabbit | poly | 1:100 | A11008 | 2nd antibody |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Kang, E.Y.; Byeon, J.; Jung, S.-h.; Hwang, C. Embossed Membranes with Vascular Patterns Guide Vascularization in a 3D Tissue Model. Polymers 2019, 11, 792. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050792
Hong S, Kang EY, Byeon J, Jung S-h, Hwang C. Embossed Membranes with Vascular Patterns Guide Vascularization in a 3D Tissue Model. Polymers. 2019; 11(5):792. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050792
Chicago/Turabian StyleHong, Soyoung, Eun Young Kang, Jaehee Byeon, Sung-ho Jung, and Changmo Hwang. 2019. "Embossed Membranes with Vascular Patterns Guide Vascularization in a 3D Tissue Model" Polymers 11, no. 5: 792. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050792