Extraction of Cellulose Nanofibers via Eco-friendly Supercritical Carbon Dioxide Treatment Followed by Mild Acid Hydrolysis and the Fabrication of Cellulose Nanopapers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
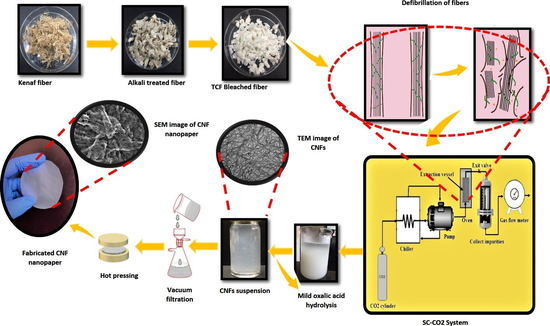
2.2. Isolation of CNFs via Supercritical Carbon Dioxide Assisted Mild Acid Hydrolysis
2.3. Fabrication of Cellulose Nanopaper Using Extracted CNFs Suspension
2.4. Characterization
2.4.1. X-Ray Diffraction (XRD)
2.4.2. Scanning Electron Microscopy (SEM) Analysis
2.4.3. Transmission Electron Microscopy (TEM) Analysis and Evaluation of Surface Area of the Extracted Cellulose Nanofibers.
2.4.4. Zeta Potential Measurements
2.4.5. Evaluation of the Mechanical Properties of the Fabricated Cellulose Nanopaper
3. Results and Discussion
3.1. Morphology of the Extracted Cellulose Nanofibers
3.2. FT-IR Studies
3.3. X-Ray Diffraction XRD Analysis
3.4. Stability of the Extracted CNFs Suspension via SC-CO2 Assisted Mild Acid Hydrolysis
3.5. Thermogravimetric Analysis (TGA)
3.6. Morphology of the Treated and Untreated Fibers
3.7. Strategy for the Fabrication of Cellulose Nanopaper
3.8. Morphology of the Fabricated Cellulose Nanopaper
3.9. Mechanical Strength of the Fabricated Cellulose Nanopaper
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Khiari, R. Valorization of agricultural residues for cellulose nanofibrils production and their use in nanocomposite manufacturing. Int. J. Polym. Sci. 2017, 2017, 6361245. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Passos de Oliveira Santos, R.; Fernanda Rossi, P.; Ramos, L.; Frollini, E. Renewable resources and a recycled polymer as raw materials: Mats from electrospinning of lignocellulosic biomass and PET solutions. Polymers 2018, 10, 538. [Google Scholar] [CrossRef]
- Li, W.; Trosien, S.; Schenderlein, H.; Graf, M.; Biesalski, M. Preparation of photochromic paper, using fiber-attached spiropyran polymer networks. RSC Adv. 2016, 6, 109514–109518. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.J.; Li, D.; Cheng, Y.L.; Adhikari, B. Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp. Carbohydr. Polym. 2014, 102, 136–143. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Chen, W.; Abe, K.; Uetani, K.; Yu, H.; Liu, Y.; Yano, H. Individual cotton cellulose nanofibers: Pretreatment and fibrillation technique. Cellulose 2014, 21, 1517–1528. [Google Scholar] [CrossRef]
- Fortunati, E.; Luzi, F.; Jiménez, A.; Gopakumar, D.A.; Puglia, D.; Thomas, S.; Kenny, J.M.; Chiralt, A.; Torre, L. Revalorization of sunflower stalks as novel sources of cellulose nanofibrils and nanocrystals and their effect on wheat gluten bionanocomposite properties. Carbohydr. Polym. 2016, 149, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Uetani, K.; Yano, H. Nanofibrillation of wood pulp using a high-speed blender. Biomacromolecules 2010, 12, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.A.; Heish, Y.T.; Tsai, T.Y.; Huang, C.F. TEMPO-oxidized pulp as an efficient and recyclable sorbent to remove paraquat from water. Cellulose 2015, 22, 3261–3274. [Google Scholar] [CrossRef]
- Huang, C.F.; Chen, J.K.; Tsai, T.Y.; Hsieh, Y.A.; Lin, K.Y.A. Dual-functionalized cellulose nanofibrils prepared through TEMPO-mediated oxidation and surface-initiated ATRP. Polymer 2015, 72, 395–405. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Kondo, T.; Kose, R.; Naito, H.; Kasai, W. Aqueous counter collision using paired water jets as a novel means of preparing bio-nanofibers. Carbohydr. Polym. 2014, 112, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Chirayil, C.J.; Mathew, L.; Thomas, S. Review of recent research in nano cellulose preparation from different lignocellulosic fibers. Rev. Adv. Mater. Sci. 2014, 37, 20–28. [Google Scholar]
- Abdul Khalil, H.P.S.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Tajarudin, H.A.; Paridah, M.T.; Rizal, S. Microbial-induced CaCO3filled seaweed-based film for green plasticulture application. J. Clean. Prod. 2018, 199, 150–163. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, H.M.; Tsao, G.T. Pretreatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnol. Prog. 1998, 14, 890–896. [Google Scholar] [CrossRef]
- Benito-Román, O.; Rodríguez-Perrino, M.; Sanz, M.T.; Melgosa, R.; Beltrán, S. Supercritical carbon dioxide extraction of quinoa oil: Study of the influence of process parameters on the extraction yield and oil quality. J. Supercrit. Fluids 2018, 139, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuneable textile cleaning and disinfection process based on supercritical CO2 and Pickering emulsions. J. Supercrit. Fluids 2016, 118, 128–139. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, J.; Yan, J.; Zheng, L. An industrial scale multiple supercritical carbon dioxide apparatus and its eco-friendly dyeing production. J. CO2 Util. 2016, 16, 272–281. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Pai, A.R.; Pottathara, Y.B.; Pasquini, D.; Carlos De Morais, L.; Luke, M.; Kalarikkal, N.; Grohens, Y.; Thomas, S. Cellulose Nanofiber-Based Polyaniline Flexible Papers as Sustainable Microwave Absorbers in the X-Band. ACS Appl. Mater. Interfaces 2018, 10, 20032–20043. [Google Scholar] [CrossRef] [PubMed]
- Gopakumar, D.A.; Pasquini, D.; Henrique, M.A.; De Morais, L.C.; Grohens, Y.; Thomas, S. Meldrum’s acid modified cellulose nanofiber-based polyvinylidene fluoride microfiltration membrane for dye water treatment and nanoparticle removal. ACS Sustain. Chem. Eng. 2017, 5, 2026–2033. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, H.M.; Wen, J.; Cao, N.; Yu, X.; Tsao, G.T. Supercritical carbon dioxide explosion as a pretreatment for cellulose hydrolysis. Biotechnol. Lett. 1995, 17, 845–850. [Google Scholar] [CrossRef]
- Kim, K.H.; Hong, J. Supercritical CO2 pretreatment of lignocellulose enhances enzymatic cellulose hydrolysis. Bioresour. Technol. 2001, 77, 139–144. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S. Enhanced enzymatic cellulose hydrolysis by subcritical carbon dioxide pretreatment of sugarcane bagasse. Bioresour. Technol. 2014, 158, 161–165. [Google Scholar] [CrossRef]
- Cruz-Espinoza, J.E.; Orduña-Díaz, A.; Rosales-Perez, M.; Zaca-Morán, O.; Delgado-Macuil, R.; Gayou, V.L.; Rojas-López, M. FTIR analysis of phenolic extracts from Moringa oleifera leaves. J. Biom. Biostat. 2012. [Google Scholar]
- Saelee, K.; Yingkamhaeng, N.; Nimchua, T.; Sukyai, P. An environmentally friendly xylanase-assisted pretreatment for cellulose nanofibrils isolation from sugarcane bagasse by high-pressure homogenization. Ind. Crops Prod. 2016, 82, 149–160. [Google Scholar] [CrossRef]
- Esmail, F.A.; Ghazy, M.B.; Owda, M.E.; El-Zawawy, W.K.; Al-Maadeed, M.A. Extraction and characterization of Nanocellulose obtained from sugarcane bagasse as agro-waste. J. Adv. Chem. 2018, 12, 4256–4264. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- González, I.; Alcalà, M.; Chinga-Carrasco, G.; Vilaseca, F.; Boufi, S.; Mutjé, P. From paper to nanopaper: Evolution of mechanical and physical properties. Cellulose 2014, 21, 2599–2609. [Google Scholar] [CrossRef]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothen, L.A.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S.S. Environmental friendly method for the extraction of coir fiber and isolation of nanofiber. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Ioelovich, M. Characterization of Various Kinds of Nanocellulose. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH Verlag GmbH and Co. KGaA: Weinheim, Germany, 2017; ISBN 9783527689972. [Google Scholar]
- Lee, S.Y.; Chun, S.J.; Kang, I.A.; Park, J.Y. Preparation of cellulose nanofibrils by high-pressure homogenizer and cellulose-based composite films. J. Ind. Eng. Chem. 2009, 15, 50–55. [Google Scholar] [CrossRef]
- Taiwo, O.A.; Razali, N.; Mohammad Rawi, N.F.; Razak, N.; Mohd Nadzri, N.W.; Mohamad Kassim, M.H.; Ibrahim, M.; Hossain, M.S.; Mohd Mahadar, M. Influence of Acid Hydrolysis Reaction Time on the Isolation of Cellulose Nanowhiskers from Oil Palm Empty Fruit Bunch Microcrystalline Cellulose. BioResources 2017, 12, 6773–6788. [Google Scholar]
- Saurabh, C.K.; Dungani, R.; Owolabi, A.F.; Atiqah, N.S.; Zaidon, A.; Aprilia, N.A.S.; Zaidul, I.S.M.; Abdul Khalil, H.P.S. Effect of hydrolysis treatment on cellulose nanowhiskers from oil palm (Elaeis guineesis) fronds: Morphology, chemical, crystallinity, and thermal characteristics. BioResources 2016, 11, 6742–6755. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi Júnior, H.L.; Zattera, A.J. Native cellulose: Structure, characterization and thermal properties. Materials (Basel) 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Hornsby, P.R.; Hinrichsen, E.; Tarverdi, K. Preparation and properties of polypropylene composites reinforced with wheat and flax straw fibers: Part II Analysis of composite microstructure and mechanical properties. J. Mater. Sci. 1997, 32, 1009–1015. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zhou, Q.; Ikkala, O.; Berglund, L.A. Strong and tough cellulose nanopaper with high specific surface area and porosity. Biomacromolecules 2011, 12, 3638–3644. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.J.; Lee, S.Y.; Doh, G.H.; Lee, S.; Kim, J.H. Preparation of ultra strength nanopapers using cellulose nanofibrils. J. Ind. Eng. Chem. 2011, 17, 521–526. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindström, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Hervy, M.; Santmarti, A.; Lahtinen, P.; Tammelin, T.; Lee, K.Y. Sample geometry dependency on the measured tensile properties of cellulose nanopapers. Mater. Des. 2017, 121, 421–429. [Google Scholar] [CrossRef]
- Liu, A.; Walther, A.; Ikkala, O.; Belova, L.; Berglund, L.A. Clay nanopaper with tough cellulose nanofiber matrix for fire retardancy and gas barrier functions. Biomacromolecules 2011, 12, 633–641. [Google Scholar] [CrossRef] [PubMed]










| Sample Name | Tensile Strength (MPa) | References |
|---|---|---|
| CNF nanopaper from Kenaf fiber | 75.7 | This work |
| Cellulose powder (KC Flock, W-50) after 4 passes through high pressure homogenizer | 71.3 | [43] |
| Nanofibrils from wood pulp fibers | 129.0 | [44] |
| Kraftpulp through a high shear stone grinder (Masuko supermasscolloider) | 168.0 | [45] |
| Clay nanopaper | 124.0 | [46] |
| Enzymatic Cellulose Nanofibrils (NFC) nanopaper from Softwood sulphite pulp fibers | 25.0 | [42] |
| CNF Content | Density (m3/kg) | Bulk (kg/m3) | Tensile Strength (MPa) | Tensile Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| 0.1% CNF | 560 ± 2.1 | 1786 | 37.0 | 845.9 | 16.092 |
| 0.2% CNF | 574 ± 1.5 | 1742 | 58.6 | 1728.1 | 18.664 |
| 0.3% CNF | 590 ± 1.9 | 1695 | 75.7 | 1885.8 | 22.744 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atiqah, M.S.N.; Gopakumar, D.A.; F. A. T., O.; Pottathara, Y.B.; Rizal, S.; Aprilia, N.A.S.; Hermawan, D.; Paridah, M.T.T.; Thomas, S.; H. P. S., A.K. Extraction of Cellulose Nanofibers via Eco-friendly Supercritical Carbon Dioxide Treatment Followed by Mild Acid Hydrolysis and the Fabrication of Cellulose Nanopapers. Polymers 2019, 11, 1813. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11111813
Atiqah MSN, Gopakumar DA, F. A. T. O, Pottathara YB, Rizal S, Aprilia NAS, Hermawan D, Paridah MTT, Thomas S, H. P. S. AK. Extraction of Cellulose Nanofibers via Eco-friendly Supercritical Carbon Dioxide Treatment Followed by Mild Acid Hydrolysis and the Fabrication of Cellulose Nanopapers. Polymers. 2019; 11(11):1813. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11111813
Chicago/Turabian StyleAtiqah, M. S. Nurul, Deepu A. Gopakumar, Owolabi F. A. T., Yasir Beeran Pottathara, Samsul Rizal, N. A. Sri Aprilia, D. Hermawan, M. T. T. Paridah, Sabu Thomas, and Abdul Khalil H. P. S. 2019. "Extraction of Cellulose Nanofibers via Eco-friendly Supercritical Carbon Dioxide Treatment Followed by Mild Acid Hydrolysis and the Fabrication of Cellulose Nanopapers" Polymers 11, no. 11: 1813. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11111813