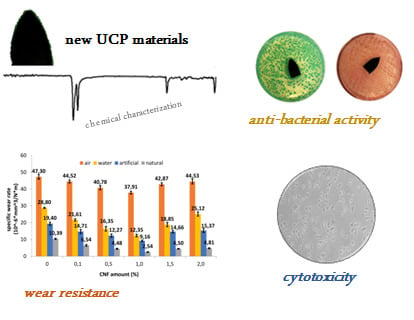
Wear Resistant Nanocomposites Based on Biomedical Grade UHMWPE Paraffin Oil and Carbon Nano-Filler: Preliminary Biocompatibility and Antibacterial Activity Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Characterization
2.2. Fourier Transform Infrared (ATR/FTIR) Spectroscopy
2.3. Fourier Transform Infrared (ATR/FTIR) Spectroscopy
2.4. Antibacterial Activity
2.5. Cytotoxicity
3. Results and Discussion
3.1. Wear Behavior
3.2. Fourier Transform Infrared (ATR/FTIR) Spectroscopy
3.3. Bioactivity Test
3.4. Antibacterial Activity
3.5. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gomez-Barrena, E.; Puertolas, J.-A.; Munuera, L.; Konttinen, Y.T. Update on UHMWPE research from the bench to the bedside. ActaOrthop 2008, 79, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chakravartula, A.; Pruitt, L.; Komvopoulos, K. Tribological and Nanomechanical Properties of Unmodified and Crosslinked Ultra-High Molecular Weight Polyethylene for Total Joint Replacements. J. Tribol. 2004, 126, 386. [Google Scholar] [CrossRef]
- Ge, S.; Kang, X.; Zhao, Y. One-year biodegradation study of UHMWPE as artificial joint materials: Variation of chemical structure and effect on friction and wear behavior. Wear 2011, 271, 2354–2363. [Google Scholar] [CrossRef]
- Yousef, S.; Visco, A.M.; Galtieri, G.; Nocita, D.; Espro, C. Wear behaviour of UHMWPE reinforced by carbon nanofiller and paraffin oil for joint replacement. Mater. Sci. Eng. C 2017, 73, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Visco, A.; Yousef, S.; Galtieri, G.; Nocita, D.; Njuguna, J. Thermal, Mechanical and Rheological Behaviors of Nanocomposites Based on UHMWPE/Paraffin Oil/Carbon Nanofiller Obtained by Using Different Dispersion Techniques. JOM-Springer 2016, 68, 1078–1089. [Google Scholar] [CrossRef] [Green Version]
- Atwood, S.A.; van Citters, D.W.; Patten, E.W.; Furmanski, J.; Ries, M.D.; Pruitt, L.A. Tradeoffs amongst fatigue, wear, and oxidation resistance of cross-linked ultra-high molecular weight polyethylene. J. Mech. Behav. Biomed. Mater. 2011, 4, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.; Fencl, R.M.; Carroll, M.; Collins, T. The relative influence of five variables on the in vitro wear rate of uncrosslinked UHMWPE acetabular cup liners. Biomaterials 2003, 24, 1925–1935. [Google Scholar] [CrossRef]
- Bruck, A.L.; KanagaKaruppiah, K.S.; Sundararajan, S.; Wang, J.; Lin, Z. Friction and wear behavior of ultrahigh molecular weight polyethylene as a function of crystallinity in the presence of the phospholipid dipalmitoyl phosphatidylcholine. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2010, 93B, 351–358. [Google Scholar] [CrossRef]
- Visco, A.M.; Campo, N. Properties of nanocomposites based on polyethylene (UHMWPE) and carbon nanotubes mixed by high energy ball milling and UV-source irradiated. Int. J. Polym. Anal. Charact. 2012, 17, 144–157. [Google Scholar]
- Muratoglu, O.K.; Bragdon, C.R.; O’Connor, D.O.; Jasty, M.; Harris, W.H.; Gul, R.; McGarry, F. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials 1999, 20, 1463–1470. [Google Scholar] [CrossRef]
- Visco, A.M.; Campo, N.; Torrisi, L.; Cristani, M.; Trombetta, D.; Saija, A. Electron beam irradiated UHMWPE: Degrading action of air and hyaluronic acid. Bio-Med. Mater. Eng. 2008, 18, 137–148. [Google Scholar] [CrossRef]
- Gul, R.M. The effects of peroxide content on the wear behavior, microstructure and mechanical properties of peroxide cross-linked UHMWPE used in total hip replacement. J. Mater. Sci. Mater. Med. 2008, 19, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.R.; Cicek, R.Z. Silanecrosslinked polyethylene for prosthetic applications Part I. Certain physical and mechanical properties related to the nature of the material. Biomaterials 1983, 4, 267–275. [Google Scholar] [CrossRef]
- Atkinson, J.R.; Cicek, R.Z. Silanecrosslinked polyethylene for prosthetic applications: II. Creep and wear behaviour and a preliminary moulding test. Biomaterials 1984, 5, 326–335. [Google Scholar] [CrossRef]
- Oral, E.; GodleskiBeckos, C.; Malhi, A.S.; Muratoglu, O.K. The effects of high dose irradiation on the cross-linking of vitamin E-blended ultrahigh molecular weight polyethylene. Biomaterials 2008, 29, 3557–3560. [Google Scholar] [CrossRef] [Green Version]
- Visco, A.M.; Campo, N.; Brancato, V.; Trimarchi, M. Influence of thea-tocopherol load and the annealing treatment on the wear resistance of biomedical UHMWPE radiated with electron beam. Int. J. Polym. Anal. Charact. 2013, 18, 545–556. [Google Scholar] [CrossRef]
- Oral, E.; Christensen, S.D.; Malhi, A.S.; Wannomae, K.K.; Muratoglu, O.K. Wear Resistance and Mechanical Properties of Highly Cross-linked, Ultrahigh–Molecular Weight Polyethylene Doped With Vitamin E. J. Arthroplast. 2006, 21, 580–591. [Google Scholar] [CrossRef] [Green Version]
- Oral, E.; GodleskiBeckos, C.; Lozynsky, A.J.; Malhi, A.S.; Muratoglu, O.K. Improved resistance to wear and fatigue fracture in high pressure crystallized vitamin E-containing ultra-high molecular weight polyethylene. Biomaterials 2009, 30, 1870–1880. [Google Scholar] [CrossRef] [Green Version]
- Oral, E.; Doshi, B.N.; Fung, K.; O’Brien, C.; Wannomae, K.K.; Muratoglu, O.K. Chemically Cross-Linked UHMWPE With Superior Toughness. J. Orthop. Res. 2019, 37, 2182–2188. [Google Scholar] [CrossRef]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a Promising Polymer Material for Biomedical Applications: A Concise Review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Panin, S.V.; Kornienko, L.A.; Nguyen, D.A.; Alexenko, V.O.; Ivanova, L.R. Enhancement of mechanical and tribotechnical properties of polymer composites with thermoplastic UHMWPE and PEEK matrices by loading carbon nanofibers/nanotubes. In Proceedings of the 11th International Conference on Mechanics, Resource and Diagnostics of Materials and Structures, AIP Conference Proceedings Mechanics, Resource and Diagnostics of Materials and Structures (Mrdms-2017), Ekaterinburg, Russia, 11–15 December 2017; Eduard, S., Gorkunov, V.E., Sunder, R., Eds.; American Institute of Physics: College Park, MD, USA, 2017; Volume 1915, p. 030015. [Google Scholar]
- Kurtz, S.M. The UHMWPE Handbook, 1st ed.; Elsevier Academic Press: San Diego, CA, USA; London, UK, 2004. [Google Scholar]
- Puértolas, J.A.; Kurtz, S.M. Evaluation of carbon nanotubes and graphene as reinforcements for UHMWPE based composites in arthroplastic applications: A review. J. Mech. Behav. Biomed. Mater. 2014, 39, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Visco, A.; Yousef, S.; Scolaro, C.; Espro, C.; Cristani, M. Tribological Behavior of Nanocomposites Based on UHMWPE Aged in Simulated Synovial Fluid. Polymers 2018, 10, 1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorito, S.; Serafino, A.; Andreola, F.; Togna, A.; Togna, G. Toxicity and biocompatibilità of carbon nanoparticles. J. Nanosci. Nanotech. 2006, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Patnaik, A.; Sharma, R.K. Parametric optimization and three-body abrasive wear behavior of sic chopped glass fiber reinforced epoxy composites. Int. J. Compos. Mater. 2013, 3, 32–38. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Harsha, A.P.; Joyce, Y.J. Challenges associated with using bovine serum in wear testing orthopaedic biopolymers. J. Eng. Med. 2011, 225, 948–958. [Google Scholar] [CrossRef]
- Ahmadi, M.; Masoomi, M.; Safi, S.; Zabihi, O. Interfacial evaluation of epoxy/carbon nanofiber nanocomposite reinforced with glycidyl methacrylate treated UHMWPE fiber. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Barick, A.K.; Tripathy, D.K. Effect of nanofiber on material properties of vapor-grown carbon nanofiber reinforced thermoplastic polyurethane (TPU/CNF) nanocomposites prepared by melt compounding. Compos. Part. A: Appl. Sci. Manuf. 2010, 41, 1471–1482. [Google Scholar] [CrossRef]
- Houmard, M.; Nunes, E.H.M.; Vasconcelos, D.C.L.; Berthomé, G.; Joud, J.-C.; Langlet, M.; Vasconcelos, W.L. Correlation between sol–gel reactivity and wettability of silica films deposited on stainless steel. Appl. Surf. Sci. 2014, 289, 218–223. [Google Scholar] [CrossRef]
- Azam, A.M.; Ali, A.; Khan, H.; Yasin, T.; Mehmood, M.S. Analysis of degradation in UHMWPE a comparative study among the various commercial and laboratory grades UHMWPE. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016. [Google Scholar]
- Ma, H.; Zeng, J.; Realff, M.L.; Kumar, S.; Schiraldi, D.A. Processing, structure, and properties of fibers from polyester/carbon nanofiber composites. Compos. Sci. Technol. 2003, 63, 1617–1628. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kokubo, T.; Yamamuro, T. Mechanism of apatite formation on CaOSiO2P2O5 glasses in a simulated body fluid. J. Non-Cryst. Solids 1992, 143, 84–92. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Poggetto, G.D.; Crescente, G.; Piccolella, S.; Pacifico, S. New SiO2/Caffeic acid hybrid materials: Synthesis, spectroscopic characterization, and bioactivity. Materials 2020, 13, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, Y.L.; Jia, S.R.; Huang, Y.; Gao, C.; Wan, Y.Z. Hydroxyapatite/bacterial cellulose composites synthesized via a biomimetic route. Mater. Lett. 2006, 60, 1710–1713. [Google Scholar]
- Catauro, M.; Tranquillo, E.; Poggetto, G.D.; Pasquali, M.; Dell’Era, A.; VecchioCiprioti, S. Influence of the heat treatment on the particles size and on the crystalline phase of TiO2 synthesized by the sol-gel method. Mataerials 2018, 11, 2364. [Google Scholar] [CrossRef] [Green Version]
- Klee, W.E.; Engel, G. IR spectra of the phosphate ions in various apatites. J. Inorg. Nuclear Chem. 1970, 32, 1837–1843. [Google Scholar] [CrossRef]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 25, 721–734. [Google Scholar] [CrossRef]
- Patlolla, A.; Knighten, B.; Tchounwou, P. Multi-walled carbon nanotubes induce cytotoxicity, genotoxicity and apoptosis in normal human dermal fibroblast cells. Ethn Dis. 2010, 20 (Suppl. 1), S1–65–72. [Google Scholar]
- Catauro, M.; Papale, F.; Bollino, F.; Picolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/quercetin sol-gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, L.; Roviello, G.; Tarallo, O.; Borbone, F.; Ferone, C.; Colangelo, F.; Catauro, M.; Cioffi, R. Synthesis and characterizations of melamine-based epoxy resins. Int. J. Mol. Sci. 2013, 14, 18200–18214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacifico, S.; Piccolella, S.; Papale, F.; Nocera, P.; Lettieri, A.; Catauro, M. A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma. J. Funct. Foods 2016, 20, 253–266. [Google Scholar] [CrossRef]
- Gazzano, E.; Bracco, P.; Bistolfi, A.; Aldieri, E.; Ghigo, D.; Boffano, M.; Costa, L.; del Prever, E.B. Ultra high molecular weight polyethylene is cytotoxic and causes oxidative stress, even when modified. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. 2), 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, K.; Garcia, A.; Sani, M.-A.; Diaz, D.; Dubey, V.; Clayton, D.; Poggetto, G.D.; Cornelius, F.; Payne, R.J.; Separovic, F.; et al. Interaction of N-terminal peptide analogues of the Na+,K+-ATPase with membranes. BBA—Biomembranes 2018, 1860, 1282–1291. [Google Scholar] [CrossRef]
- Kiventer, J.; Lancellotti, I.; Catauro, M.; Poggetto, F.D.; Leonelli, C.; Illikainen, M. Alkali activation as new option for gold mine tailings inertization. J. Clean. Prod. 2018, 187, 76–84. [Google Scholar] [CrossRef]
- Samal, S.; Kolinova, M.; Rahier, H.; Poggetto, G.D.; Blanco, I. Investigation of the internal structure of fiber reinforced geopolymer composite under mechanical impact: A micro computed tomography (μCT) study. Appl. Sci. 2019, 9, 516. [Google Scholar] [CrossRef] [Green Version]
- Torrisi, L.; Visco, A.; Campo, N. Pulsed laser treatments of polyethylene films. Nucl. Instrum. Meth. B 2010, 268, 3117–3121. [Google Scholar] [CrossRef]
- Cioffi, R.; Aronne, P.P.A.; Catauro, M.; Quattroni, G. Glass-Ceramics from fly ash with added Li2O. J. Eur. Ceram. Soc. 1994, 13, 143–148. [Google Scholar] [CrossRef]





| Sample Code | Density (g/mL) |
|---|---|
| UP | 0.866 ± 0.001 |
| UPC 0.1 wt % | 0.864 ± 0.001 |
| UPC 0.5 wt % | 0.862 ± 0.002 |
| UPC 1.0 wt % | 0.862 ± 0.004 |
| UPC 1.5 wt % | 0.863 ± 0.002 |
| UPC 2.0 wt % | 0.865 ± 0.002 |
| Ion | Concentration/mol m3 | |
|---|---|---|
| SBF | Human Blood Plasma | |
| Na+ | 142.0 | 142.0 |
| K+ | 5.0 | 5.0 |
| Mg2+ | 1.5 | 1.5 |
| Ca2+ | 2.5 | 2.5 |
| Cl− | 147.8 | 103.0 |
| HCO3− | 4.2 | 27.0 |
| HPO42− | 1.0 | 1.0 |
| SO42− | 0.5 | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catauro, M.; Scolaro, C.; Dal Poggetto, G.; Pacifico, S.; Visco, A. Wear Resistant Nanocomposites Based on Biomedical Grade UHMWPE Paraffin Oil and Carbon Nano-Filler: Preliminary Biocompatibility and Antibacterial Activity Investigation. Polymers 2020, 12, 978. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040978
Catauro M, Scolaro C, Dal Poggetto G, Pacifico S, Visco A. Wear Resistant Nanocomposites Based on Biomedical Grade UHMWPE Paraffin Oil and Carbon Nano-Filler: Preliminary Biocompatibility and Antibacterial Activity Investigation. Polymers. 2020; 12(4):978. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040978
Chicago/Turabian StyleCatauro, Michelina, Cristina Scolaro, Giovanni Dal Poggetto, Severina Pacifico, and Annamaria Visco. 2020. "Wear Resistant Nanocomposites Based on Biomedical Grade UHMWPE Paraffin Oil and Carbon Nano-Filler: Preliminary Biocompatibility and Antibacterial Activity Investigation" Polymers 12, no. 4: 978. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12040978