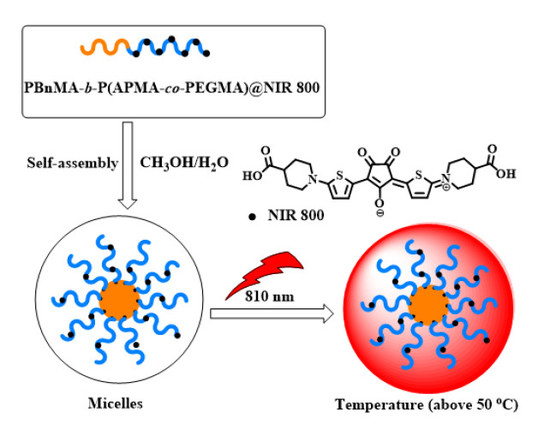
Construction of NIR Light Controlled Micelles with Photothermal Conversion Property: Poly(poly(ethylene glycol)methyl ether methacrylate) (PPEGMA) as Hydrophilic Block and Ketocyanine Dye as NIR Photothermal Conversion Agent
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of BAPMA
2.3. Synthesis of (Z)-5-(5-(4-carboxypiperidin-1-ium-1-ylidene)thiophen-2(5H)-ylidene)-2-(5-(4-carboxypiperidin-1-yl)thiophen-2-yl)-3,4-dioxocyclopent-1-en-1-olate (NIR 800)
2.4. General Procedure for RAFT Polymerization of BnMA
2.5. General Procedure for Synthesis of PBnMA-b-P(BAPMA-co-PEGMA)
2.6. General Procedure for Synthesis of PBnMA-b-P(APMA-co-PEGMA)
2.7. Measurement of Critical Micelle Concentration (CMC) of PBnMA-b-P(APMA-co-PEGMA)
2.8. Post-Modification of PBnMA-b-P(APMA-co-PEGMA) with NIR 800
2.9. Self-Assembly of Block Copolymer and Post-Modified Block Copolymer
2.10. Measurement of NIR Photothermal Conversion Performance of NIR 800 and Post-Modified Micelles
2.11. Experiment of Cytotoxicity
2.12. Characterizations
3. Results and Discussion
3.1. 1H NMR of BAPMA and NIR 800
3.2. NIR Photothermal Conversion Performance of NIR 800
3.3. Synthesis and Self-Assembly of Post-Modified Copolymer PBnMA-b-P(APMA-co-PEGMA) @NIR 800
3.4. NIR Photothermal Conversion Performance of Post-Modified Micelles from PBnMA-b-P(APMA-co-PEGMA)@NIR 800
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tregubov, A.A.; Nikitin, P.I.; Nikitin, M.P. Advanced smart nanomaterials with integrated logic-gating and biocomputing: Dawn of theranostic nanorobots. Chem. Rev. 2018, 118, 10294–10348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sherbini, A.A.; Ghannam, H.E.A.; El-Ghanam, G.M.A.; El-Ella, A.A.; Youssef, A.M. Utilization of chitosan/Ag bionanocomposites as eco-friendly photocatalytic reactor for bactericidal effect and heavy metals removal. Heliyon 2019, 5, e01980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adewale, O.B.; Davids, H.; Cairncross, L.; Roux, S. Toxicological behavior of gold nanoparticles on various models: Influence of physicochemical properties and other factors. Int. J. Toxicol. 2019, 38, 357–384. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Ahmad, N.; Nelayah, J.; Wilhelm, C.; Silva, A.; Gazeau, F.; Alloyeau, D. Monitoring the dynamics of cell-derived extracellular vesicles at the nanoscale by liquid-cell transmission electron microscopy. Nanoscale 2018, 10, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ji, D.; Zhang, Y.; Gao, X.; Xu, P.; Li, X.; Liu, C.C.; Wen, W. Detection of phenylketonuria markers using a ZIF-67 encapsulated PtPd alloy nanoparticle (PtPd@ZIF-67)-based disposable electrochemical microsensor. ACS Appl. Mater. Interfaces 2019, 11, 20734–20742. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Lin, J.; Kao, S.; Liao, Z.; Zhu, J.; Huang, X.; Zhang, R.; Xin, H.L. Atomistic defect makes a phase plate for the generation and high-angular splitting of electron vortex beams. ACS Nano 2019, 13, 3964–3970. [Google Scholar] [CrossRef]
- Delaittre, G.; Dire, C.; Rieger, J.; Putaux, J.L.; Charleux, B. Formation of polymer vesicles by simultaneous chain growth and self-assembly of amphiphilic block copolymers. Chem. Commun. 2009, 20, 2887–2889. [Google Scholar] [CrossRef]
- Vaculikova, E.; Grunwaldova, V.; Kral, V.; Dohnal, J.; Jampilek, J. Preparation of candesartan and atorvastatin nanoparticles by solvent evaporation. Molecules 2012, 17, 13221–13234. [Google Scholar] [CrossRef]
- Alaboalirat, M.; Qi, L.Q.; Arrington, K.J.; Qian, S.; Keum, J.K.; Mei, H.; Littrell, K.C.; Sumpter, B.G.; Carrillo, J.M.Y.; Verduzco, R.; et al. Amphiphilic bottlebrush block copolymers: Analysis of aqueous self-assembly by small-angle neutron scattering and surface tension measurements. Macromolecules 2019, 52, 465–476. [Google Scholar] [CrossRef]
- Lee, J.; Pan, J.; Chun, J.; Won, Y.Y. Unexpected conformational behavior of poly(poly(ethylene glycol) methacrylate)-poly(propylene carbonate)-poly(poly(ethylene glycol) methacrylate) (PPEGMA-PPC-PPEGMA) amphiphilic block copolymers in micellar solution and at the air-water interface. J. Colloid Interface Sci. 2020, 566, 304–315. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, P.; Yu, J.; Jiang, H.; Gao, P.; Ma, J. Enhanced permeability and fouling-resistant capacity of poly (vinylidene fluoride) ultrafiltration membrane based on the PPG-co-PEG-co-PPG copolymer with two hydrophobic terminals and one hydrophilic intermediate. Water Sci. Technol. 2019, 79, 2068–2078. [Google Scholar] [CrossRef]
- Yagishita, M.; Kubo, T.; Nakano, T.; Shiraishi, F.; Tanigawa, T.; Naito, T.; Sano, T.; Nakayama, S.F.; Nakajima, D.; Otsuka, K. Efficient extraction of estrogen receptor-active compounds from environmental surface water via a receptor-mimic adsorbent, a hydrophilic PEG-based molecularly imprinted polymer. Chemosphere 2019, 217, 204–212. [Google Scholar] [CrossRef]
- Liu, X.; Chen, B.; Li, X.; Zhang, L.; Xu, Y.; Liu, Z.; Cheng, Z.; Zhu, X. Self-assembly of BODIPY based pH-sensitive near-infrared polymeric micelles for drug controlled delivery and fluorescence imaging applications. Nanoscale 2015, 7, 16399–16416. [Google Scholar] [CrossRef]
- Deng, F.; Zhou, H.; Chen, J.; Huang, H.; Tian, J.; Wen, Y.; Huang, Q.; Liu, M.; Zhang, X.; Wei, Y. Surface PEGylation and biological imaging of fluorescent Tb (3+)-doped layered double hydroxides through the photoinduced RAFT polymerization. J. Colloid Interface Sci. 2018, 532, 641–649. [Google Scholar] [CrossRef]
- Dong, J.; Liu, M.; Jiang, R.; Huang, H.; Wan, Q.; Wen, Y.; Tian, J.; Dai, Y.; Zhang, X.; Wei, Y. Synthesis and biological imaging of cross-linked fluorescent polymeric nanoparticles with aggregation-induced emission characteristics based on the combination of RAFT polymerization and the biginelli reaction. J. Colloid Interface Sci. 2018, 528, 192–199. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Huang, Q.; Jiang, R.; Huang, H.; Deng, F.; Wen, Y.; Tian, J.; Zhang, X.; Wei, Y. A novel strategy for fabrication of fluorescent hydroxyapatite based polymer composites through the combination of surface ligand exchange and self-catalyzed ATRP. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 518–525. [Google Scholar] [CrossRef]
- Huang, D.; Qian, H.; Qiao, H.; Chen, W.; Jan, F.J.; Zhong, Z. Bioresponsive functional nanogels as an emerging platform for cancer therapy. Expert. Opin. Drug Deliv. 2018, 15, 703–716. [Google Scholar] [CrossRef]
- Li, S.; Tian, M.; Wang, J.; Du, F.; Li, L.; Xue, Z. Poly (ethylene oxide)-based block copolymer electrolytes formed via ligand-free iron-mediated atom transfer radical polymerization. Polymers 2020, 12, 763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieswandt, K.; Georgopanos, P.; Abetz, C.; Filiz, V.; Abetz, V. Synthesis of poly(3-vinylpyridine)-block-polystyrene diblock copolymers via surfactant-free RAFT emulsion polymerization. Materials 2019, 12, 3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Sun, M.; Sun, J.; Hu, J.; Wang, Z.; Guo, J.; Gao, W. Polymerization induced self-assembly of a site-specific interferon alpha-block copolymer conjugate into micelles with remarkably enhanced pharmacology. J. Am. Chem. Soc. 2018, 140, 10435–10438. [Google Scholar] [CrossRef] [PubMed]
- Bastakoti, B.P.; Perez-Mercader, J. Facile one-pot synthesis of functional giant polymeric vesicles controlled by oscillatory chemistry. Angew. Chem. Int. Ed. Engl. 2017, 56, 12086–12091. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; An, Z.; Wu, P. Scalable preparation of alternating block copolymer particles with inverse bicontinuous mesophases. Nat. Commun. 2019, 10, 1397–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Khramtsov, V.V. In vivo EPR assessment of pH, pO2, redox status, and concentrations of phosphate and glutathione in the tumor microenvironment. J. Vis. Exp. 2018, 133, e56624. [Google Scholar]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR effect: Combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef] [PubMed]
- Bort, G.; Lux, F.; Dufort, S.; Cremillieux, Y.; Verry, C.; Tillement, O. EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic AGuIX nanoparticles. Theranostics 2020, 10, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Dong, A.J.; Guo, R.W.; Yang, M.Y.; Deng, L.D.; Zhang, J.H. DOX/ICG coencapsulated liposome-coated thermosensitive nanogels for NIR-triggered simultaneous drug release and photothermal effect. ACS Biomater. Sci. Eng. 2018, 4, 2424–2434. [Google Scholar] [CrossRef]
- Gao, H.; Bi, Y.; Wang, X.; Wang, M.; Zhou, M.; Lu, H.; Gao, J.; Chen, J.; Hu, Y. Near-infrared guided thermal-responsive nanomedicine against orthotopic superficial bladder cancer. ACS Biomater. Sci. Eng. 2017, 3, 3628–3634. [Google Scholar] [CrossRef]
- Zheng, T.; Li, G.G.; Zhou, F.; Wu, R.; Zhu, J.J.; Wang, H. Gold-nanosponge-based multistimuli-responsive drug vehicles for targeted chemo-photothermal therapy. Adv. Mater. 2016, 28, 8218–8226. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Wang, Y.; Zhu, H.; Li, F.; Shen, Y.; Guo, S. A new NIR-triggered doxorubicin and photosensitizer indocyanine green co-delivery system for enhanced multidrug resistant cancer treatment through simultaneous chemo/photothermal/photodynamic therapy. Acta Biomater. 2017, 59, 170–180. [Google Scholar] [CrossRef]
- Wang, H.; Mukherjee, S.; Yi, J.; Banerjee, P.; Chen, Q.; Zhou, S. Biocompatible chitosan-carbon dot hybrid nanogels for NIR-imaging-guided synergistic photothermal-chemo therapy. ACS Appl. Mater. Interfaces 2017, 9, 18639–18649. [Google Scholar] [CrossRef]
- Song, W.; Li, Y.; Wang, Y.; Wang, D.; He, D.; Chen, W.; Yin, W.; Yang, W. Indocyanine green-loaded gold nanoflowers@two layers of silica nanocomposites for photothermal and photodynamic therapy of oral carcinoma. J. Biomed. Nanotechnol. 2017, 13, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Hou, M.; Gao, Y.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Indocyanine green-modified hollow mesoporous Prussian blue nanoparticles loading doxorubicin for fluorescence-guided tri-modal combination therapy of cancer. Nanoscale 2019, 11, 5717–5731. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Si, W.; Huang, C.; Ding, K.; Huang, W.; Chen, P.; Zhang, Q.; Dong, X. An aza-BODIPY photosensitizer for photoacoustic and photothermal imaging guided dual modal cancer phototherapy. J. Mater. Chem. B 2017, 5, 1566–1573. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, X.; Fan, J.; Du, J.; Peng, X. Boron dipyrromethene nano-photosensitizers for anticancer phototherapies. Small 2019, 15, e1804927. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, W.; Zhang, W.; Li, C.; Sun, T.; Chen, G.; Xie, Z. Amphiphilic redox-sensitive NIR BODIPY nanoparticles for dual-mode imaging and photothermal therapy. J. Colloid Interface Sci. 2019, 536, 208–214. [Google Scholar] [CrossRef]
- Lu, M.; Kang, N.; Chen, C.; Yang, L.; Li, Y.; Hong, M.; Luo, X.; Ren, L.; Wang, X. Plasmonic enhancement of cyanine dyes for near-infrared light-triggered photodynamic/photothermal therapy and fluorescent imaging. Nanotechnology 2017, 28, 445710. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Yang, Z.; Zhang, X.; Liu, Z.; Hua, J. NIR organic dyes based on phenazine-cyanine for photoacoustic imaging-guided photothermal therapy. J. Mater. Chem. B 2018, 6, 7420–7426. [Google Scholar] [CrossRef]
- Cao, J.; Chi, J.; Xia, J.; Zhang, Y.; Han, S.; Sun, Y. Iodinated cyanine dyes for fast near-infrared-guided deep tissue synergistic phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 25720–25729. [Google Scholar] [CrossRef]
- Johnson, J.R.; Fu, N.; Arunkumar, E.; Leevy, W.M.; Gammon, S.T.; Piwnica-Worms, D.; Smith, B.D. Squaraine rotaxanes: Superior substitutes for Cy-5 in molecular probes for near-infrared fluorescence cell imaging. Angew. Chem. Int. Ed. Engl. 2007, 46, 5528–5531. [Google Scholar] [CrossRef]
- Zielichowska, A.; Saczko, J.; Garbiec, A.; Dubinska-Magiera, M.; Rossowska, J.; Surowiak, P.; Choromanska, A.; Daczewska, M.; Kulbacka, J.; Lage, H. The photodynamic effect of far-red range phthalocyanines (AlPc and Pc green) supported by electropermeabilization in human gastric adenocarcinoma cells of sensitive and resistant type. Biomed. Pharmacother. 2015, 69, 145–152. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Yu, Y.; Guo, S.; Wang, W.; Zhu, S. A cyanine-derivative photosensitizer with enhanced photostability for mitochondria-targeted photodynamic therapy. Chem. Commun. 2019, 55, 13542–13545. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, V.; Pandey, R.; Das, P.K.; Castet, F.; Blanchard-Desce, M. Linear and nonlinear optical properties of tricyanopropylidene-based merocyanine dyes: Synergistic experimental and theoretical investigations. Chemphyschem 2018, 19, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Avirah, R.R.; Jyothish, K.; Ramaiah, D. Infrared absorbing croconaine dyes: Synthesis and metal ion binding properties. J. Org. Chem. 2008, 73, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Tatsuura, S.; Furuki, M.; Sato, Y.; Iwasa, I.; Pu, L.S. Discovery of novel dyes with absorption maxima at 1.1 microm. J. Am. Chem. Soc. 2003, 125, 348–349. [Google Scholar] [CrossRef] [PubMed]
- McGarraugh, H.H.; Liu, W.; Matthews, B.P.; Smith, B.D. Croconaine rotaxane dye with 984 nm absorption: Wavelength-selective photothermal heating. Eur. J. Org. Chem. 2019, 2019, 3489–3494. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Foley, J.W. A new water-soluble near-infrared croconium dye. Dyes Pigments 2008, 78, 60–64. [Google Scholar] [CrossRef]
- Liu, L.; Liu, M.H.; Deng, L.L.; Lin, B.P.; Yang, H. Near-infrared chromophore functionalized soft actuator with ultrafast photoresponsive speed and superior mechanical property. J. Am. Chem. Soc. 2017, 139, 11333–11336. [Google Scholar] [CrossRef]
- Carruthers, N.I.; Spitler, J.M.; Wong, S.C.; Blythin, D.J.; Chen, X.; Shue, H.J.; She, H.S.; Lee, J.F.; Rizzo, C.; Ting, P.C.; et al. Synthesis of a series of sulfinic acid analogs of GABA and evaluation of their GABAB receptor affinities. Bioorg. Med. Chem. Lett. 1998, 8, 3059–3064. [Google Scholar] [CrossRef]
- Kus, N.; Fausto, R. Near-infrared and ultraviolet induced isomerization of crotonic acid in N2 and Xe cryomatrices: First observation of two high-energy trans C-O conformers and mechanistic insights. J. Chem. Phys. 2014, 141, 234310. [Google Scholar] [CrossRef]
- Grabska, J.; Ishigaki, M.; Bec, K.B.; Wojcik, M.J.; Ozaki, Y. Correlations between structure and near-infrared spectra of saturated and unsaturated carboxylic acids. Insight from anharmonic density functional theory calculations. J. Phys. Chem. A 2017, 121, 3437–3451. [Google Scholar] [CrossRef]
- Monajati, M.; Tavakoli, S.; Abolmaali, S.S.; Yousefi, G.; Tamaddon, A. Effect of PEGylation on assembly morphology and cellular uptake of poly ethyleneimine-cholesterol conjugates for delivery of sorafenib tosylate in hepatocellular carcinoma. Bioimpacts 2018, 8, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Basu Ray, G.; Chakraborty, I.; Moulik, S.P. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J. Colloid Interface Sci. 2006, 294, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Deng, C. Designed synthesis of a “one for two” hydrophilic magnetic amino-functionalized metal-organic framework for highly efficient enrichment of glycopeptides and phosphopeptides. Sci. Rep. 2017, 7, 1162–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Dai, J.; Zhang, G.; Zhang, Y.; Li, S.; Nie, D. Photothermal/pH dual-responsive drug delivery system of amino-terminated HBP-modified rGO and the chemo-photothermal therapy on tumor cells. Nanoscale Res. Lett. 2018, 13, 379–388. [Google Scholar] [CrossRef] [PubMed]













| Entry | R | Macro-CTA | a Mn, GPC (g/mol) | b Mn, NMR (g/mol) | a Mw/Mn |
|---|---|---|---|---|---|
| P4 | c 10/10/1/0.3 | P1:PBnMA a Mn, GPC = 2400 g/mol a Mw/Mn = 1.11 b Mn, NMR = 2400 g/mol (for P4 P7) | 9900 | 10100 | 1.08 |
| P5 | 7400 | 10700 | 1.25 | ||
| P6 | 6500 | 11200 | 1.5 | ||
| P7 | d 10/5/1/0.3 | P2:PBnMA a Mn, GPC = 3200 g/mol a Mw/Mn = 1.13 b Mn, NMR = 3000 g/mol (for P5 P8) | 6400 | 7300 | 1.14 |
| P8 | P3:PBnMA a Mn, GPC = 3600 g/mol a Mw/Mn = 1.14 b Mn, NMR = 3100 g/mol (for P6 P9) | 5600 | 8800 | 1.27 | |
| P9 | 6900 | 8100 | 1.18 |
| Sample | b Size (nm) | b PDI | ||||
|---|---|---|---|---|---|---|
| c P | c P-NH2 | c P-NH2@NIR 800 | c P | c P-NH2 | c P-NH2@NIR 800 | |
| P4: a 12-8-12 | 550 | 379 | 114 | 0.081 | 0.097 | 0.059 |
| P5:a 18-7-11 | 337 | 376 | 0.114 | 0.017 | ||
| P6:a 20-8-11 | 339 | 322 | 0.002 | 0.016 | ||
| P7: a 12-8-6 | 446 | 274 | 0.037 | 0.065 | ||
| P8:a 18-9-6 | 324 | 324 | 0.044 | 0.095 | ||
| P9:a 20-7-5 | 236 | 477 | 0.42 | 0.11 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Li, H.; Tu, K.; Zhang, L.; Cheng, Z.; Zhu, X. Construction of NIR Light Controlled Micelles with Photothermal Conversion Property: Poly(poly(ethylene glycol)methyl ether methacrylate) (PPEGMA) as Hydrophilic Block and Ketocyanine Dye as NIR Photothermal Conversion Agent. Polymers 2020, 12, 1181. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051181
Yao L, Li H, Tu K, Zhang L, Cheng Z, Zhu X. Construction of NIR Light Controlled Micelles with Photothermal Conversion Property: Poly(poly(ethylene glycol)methyl ether methacrylate) (PPEGMA) as Hydrophilic Block and Ketocyanine Dye as NIR Photothermal Conversion Agent. Polymers. 2020; 12(5):1181. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051181
Chicago/Turabian StyleYao, Lan, Haihui Li, Kai Tu, Lifen Zhang, Zhenping Cheng, and Xiulin Zhu. 2020. "Construction of NIR Light Controlled Micelles with Photothermal Conversion Property: Poly(poly(ethylene glycol)methyl ether methacrylate) (PPEGMA) as Hydrophilic Block and Ketocyanine Dye as NIR Photothermal Conversion Agent" Polymers 12, no. 5: 1181. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051181