Study on Optimization of Damping Performance and Damping Temperature Range of Silicone Rubber by Polyborosiloxane Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of LPBS/MVQ, PBS/MVQ, and HPBS/MVQ
2.3. Characterization
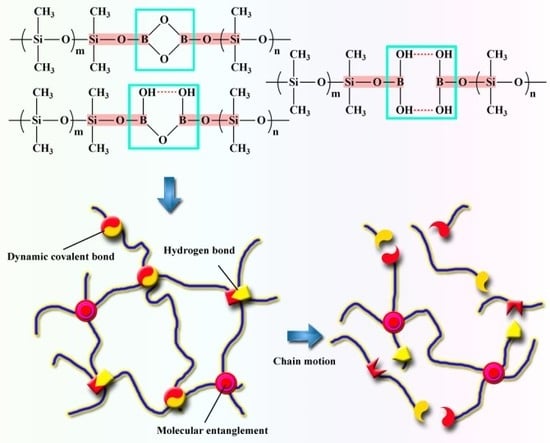
3. Results and Discussions
3.1. Analysis of PBS-Gel
3.2. Damping Properties of Composite Silicone Rubber
3.3. Mechanical Properties of Composite Silicone Rubber
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, D. Review Materials for Vibration Damping. J. Mater. Sci. 2011, 36, 5733–5737. [Google Scholar] [CrossRef]
- Adams, R.D.; Maheri, M.R. Damping in Advanced Polymer–Matrix Composites. J. Alloys Compd. 2003, 355, 126–130. [Google Scholar] [CrossRef]
- Treviso, A.; Van Genechten, B.; Mundo, D.; Tournour, M. Damping in Composite Materials: Properties and Models. Compos. Part B Eng. 2015, 78, 144–152. [Google Scholar] [CrossRef]
- Buravalla, V.; Remillat, C.; Rongong, J.; Tomlinson, G. Advances in Damping Materials and Technology. Smart Mater. Bull. 2001, 2001, 10–13. [Google Scholar] [CrossRef]
- Li, Z.; Lu, X.; Tao, G.; Guo, J.; Jiang, H. Damping Elastomer with Broad Temperature Range Based on Irregular Networks Formed by End-Linking of Hydroxyl-Terminated Poly (Dimethylsiloxane). Polym. Eng. Sci. 2016, 56, 97–102. [Google Scholar] [CrossRef]
- Sperling, L.; Fay, J. Factors which affect the glass transition and damping capability of polymers. Polym. Adv. Technol. 1991, 2, 49–56. [Google Scholar] [CrossRef]
- Yu, F.; Lu, A.; Lu, J.; Wang, Z.; Zhang, Q.; Geng, C.; Li, Z. Effect of Phenyl Content, Sample Thickness and Compression on Damping Performances of Silicone Rubber: A Study by Dynamic Mechanical Analysis and Impact Damping Test. Polym. Test. 2019, 80, 106101. [Google Scholar] [CrossRef]
- Wang, X.; Dong, W. Preparation of Graphite Oxide (GO) and the Thermal Stability of Silicone Rubber/GO Nanocomposites. Thermochim. Acta 2012, 529, 25–28. [Google Scholar] [CrossRef]
- Kole, S.; Chaki, T.K.; Bhowmick, A.K.; Tripathy, D.K. Effect of Compatibiliser, Curing Sequence and Ageing on the Thermal Stability of Silicone rubber, EPDM rubber and their blends. Polym. Degrad. Stab. 1993, 41, 109–116. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Wang, T.; Zhang, X. Damping, Thermal, and Mechanical Properties of Polyurethane Based on Poly (Tetramethylene Glycol)/Epoxy Interpenetrating Polymer Networks: Effects of Composition and Isocyanate Index. Appl. Phys. A Mater. Sci. Process. 2011, 104, 375–382. [Google Scholar] [CrossRef]
- Liu, Z.; Picken, S.J.; Besseling, N.A. Polyborosiloxanes (PBSs), Synthetic Kinetics, and Characterization. Macromolecules 2014, 47, 4531–4537. [Google Scholar] [CrossRef]
- Fukahori, Y.; Sakulkaew, K.; Busfield, J. Elastic-Viscous Transition in Tear Fracture of Rubbers. Polymer 2013, 54, 1905–1915. [Google Scholar] [CrossRef]
- Huang, G.; Jiang, L.; Li, Q. Molecular Design of Damping Rubber Based on Polyacrylate-Containing Silicone. J. Appl. Polym. Sci. 2002, 85, 746–751. [Google Scholar] [CrossRef]
- Zhang, C.; Pal, K.; Byeon, J.U.; Han, S.M.; Kim, J.K. A Study on Mechanical and Thermal Properties of Silicone Rubber/EPDM Damping Materials. J. Appl. Polym. Sci. 2011, 119, 2737–2741. [Google Scholar] [CrossRef]
- Urayama, K.; Miki, T.; Takigawa, T.; Kohjiya, S. Damping Elastomer Based on Model Irregular Networks of End-Linked Poly (Dimethylsiloxane). Chem. Mater. 2004, 16, 173–178. [Google Scholar] [CrossRef]
- Hourston, D.J.; Schäfer, F.U. Polyurethane/Polystyrene Oneshot Interpenetrating Polymer Networks with Good Damping Ability: Transition Broadening Through Crosslinking, Internetwork Grafting and Compatibilization. Polym. Adv. Technol. 1996, 7, 273–280. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayashi, M. Vibration Damping Silicone Composition. U.S. Patent No 6,498,211, 5 August 2002. [Google Scholar]
- Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Using the dynamic bond to access macroscopically responsive structurally dynamic polymers. Nat. Mater. 2011, 10, 14–27. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulié-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef]
- Wright, J.G. Process for Making Puttylike Elastic Plastic, Siloxane Derivative Composition Containing Zinc Hydroxide. U.S. Patent No 2,541,851, 13 February 1951. [Google Scholar]
- Juhasz, A.; Tasnadi, P.; Fábry, L. Impact studies on the mechanical properties of polyborosiloxane. Phys. Educ. 1984, 19, 302. [Google Scholar] [CrossRef]
- Tang, M.; Wang, W.; Xu, D.; Wang, Z. Synthesis of structure-controlled polyborosiloxanes and investigation on their viscoelastic response to molecular mass of polydimethylsiloxane triggered by both chemical and physical interactions. Ind. Eng. Chem. Res. 2016, 55, 12582–12589. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, N.; Chen, X.; He, B. Rheology of crosslinked entangled polymers: Shear stiffening in oscillatory shear. J. Appl. Polym. Sci. 2020, 137, 48421. [Google Scholar] [CrossRef]
- Sreejith, K.; Prabhakaran, P.; Laly, K.; Dimple, R.; Packirisamy, S. Vinyl-functionalized poly (borosiloxane) as precursor for SiC/SiBOC nanocomposite. Ceram. Int. 2016, 42, 15285–15293. [Google Scholar] [CrossRef]
- Atanasov, S.E.; Oldham, C.J.; Slusarski, K.A.; Taggart-Scarff, J.; Sherman, S.A.; Senecal, K.J.; Filocamo, S.F.; McAllister, Q.P.; Wetzel, E.D.; Parsons, G.N. Improved Cut-Resistance of Kevlar® Using Controlled Interface Reactions During Atomic Layer Deposition of Ultrathin (<50 Å) Inorganic Coatings. J. Mater. Chem. A 2014, 2, 17371–17379. [Google Scholar]
- Tan, L.B.; Tse, K.M.; Lee, H.P.; Tan, V.B.C.; Lim, S.P. Performance of an Advanced Combat Helmet with Different Interior Cushioning Systems in Ballistic Impact: Experiments and Finite Element Simulations. Int. J. Impact Eng. 2012, 50, 99–112. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Wells, G. Influence of Yarn Gripping on the Ballistic Performance of Woven Fabrics from Ultra-High Molecular Weight Polyethylene Fibre. Compos. Part B Eng. 2014, 62, 198–204. [Google Scholar] [CrossRef]
- Wang, S.; Xuan, S.; Wang, Y.; Xu, C.; Mao, Y.; Liu, M.; Bai, L.; Jiang, W.; Gong, X. Stretchable Polyurethane Sponge Scaffold Strengthened Shear Stiffening Polymer and Its Enhanced Safeguarding Performance. ACS Appl. Mater. Interfaces 2016, 8, 4946–4954. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Q.; Wang, Y.; Xuan, S.; Jiang, W.; Gong, X. High Strain-Rate Dynamic Mechanical Properties of Kevlar Fabrics Impregnated with Shear Thickening Fluid. Compos. Part A Appl. Sci. Manuf. 2017, 100, 161–169. [Google Scholar] [CrossRef]
- Ballard, M.; Buscall, R.; Waite, F. The Theory of Shear-Thickening Polymer Solutions. Polymer 1988, 29, 1287–1293. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Tsou, A.H. Strain Hardening of Polyethylene/Polypropylene Blends via Interfacial Reinforcement with Poly(Ethylene-co-Propylene) Comb Block Copolymers. Macromolecules 2017, 50, 2986–2995. [Google Scholar] [CrossRef]
- Tanaka, F.; Edwards, S. Viscoelastic Properties of Physically Crosslinked Networks. 1. Transient Network Theory. Macromolecules 1992, 25, 1516–1523. [Google Scholar] [CrossRef]
- Allen, F.H. Relationships between Experiment and Theory in the Study of Intermolecular Interactions, Intermolecular Interactions; Springer: Boston, MA, USA, 1998; pp. 111–120. [Google Scholar]
- Bouzid, M.; Del Gado, E. Network Topology in Soft Gels: Hardening and Softening Materials. Langmuir 2017, 34, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Plant, D.; Leevers, P. Mechanical and rheological testing to develop thermoplastic elastomer-polyborodimethylsiloxane blends for personal impact protection. Polym. Test. 2020, 86, 106477. [Google Scholar] [CrossRef]
- Kulik, V.; Boiko, A.; Bardakhanov, S.; Park, H.; Chun, H.; Lee, I. Viscoelastic Properties of Silicone Rubber with Admixture of SiO2 Nanoparticles. Mater. Sci. Eng. A 2011, 528, 5729–5732. [Google Scholar] [CrossRef]
- Laun, H.; Bung, R.; Hess, S.; Loose, W.; Hess, O.; Hahn, K.; Hädicke, E.; Hingmann, R.; Schmidt, F.; Lindner, P. Rheological and Small Angle Neutron Scattering Investigation of Shear Induced Particle Structures of Concentrated Polymer Dispersions Submitted to Plane Poiseuille and Couette Flow. J. Rheol. 1992, 36, 743–787. [Google Scholar] [CrossRef]
- Yilgor, I.; Eynur, T.; Bilgin, S.; Yilgor, E.; Wilkes, G.L. Influence of Soft Segment Molecular Weight on the Mechanical Hysteresis and Set Behavior of Silicone-Urea Copolymers with Low Hard Segment Contents. Polymer 2011, 52, 266–274. [Google Scholar] [CrossRef]










| Sample | C% | |
|---|---|---|
| 25 °C, 70 h | 75 °C, 70 h | |
| MVQ | 10 | 37 |
| 10 wt % LPBS/MVQ | 17 | 38 |
| 20 wt % LPBS/MVQ | 17 | 41 |
| 30 wt % LPBS/MVQ | 21 | 46 |
| 40 wt % LPBS/MVQ | 24 | 47 |
| 40 wt % PBS/MVQ | 19 | 46 |
| 40 wt % HPBS/MVQ | 17 | 43 |
| Samples | Tensile Modulus/MPa | Tensile Strength/MPa | Elongation at Break/% | Hardness/Shore A | |
|---|---|---|---|---|---|
| Strain-100% | Strain-300% | ||||
| MVQ | 0.49 ± 0.05 | 1.26 ± 0.10 | 8.61 ± 0.50 | 1061.32 ± 10.00 | 69.72 ± 2.00 |
| 10 wt % LPBS/MVQ | 0.43 ± 0.05 | 0.98 ± 0.10 | 7.94 ± 0.50 | 1151.70 ± 10.00 | 64.85 ± 2.00 |
| 20 wt % LPBS/MVQ | 0.35 ± 0.05 | 0.93 ± 0.10 | 6.09 ± 0.50 | 1758.25 ± 10.00 | 53.50 ± 2.00 |
| 30 wt % LPBS/MVQ | 0.28 ± 0.05 | 0.68 ± 0.10 | 5.57 ± 0.50 | 2539.00 ± 10.00 | 44.25 ± 2.00 |
| 40 wt % LPBS/MVQ | 0.20 ± 0.05 | 0.39 ± 0.10 | 5.28 ± 0.50 | 2642.00 ± 10.00 | 25.67 ± 2.00 |
| 40 wt % PBS/MVQ | 0.19 ± 0.05 | 0.44 ± 0.10 | 5.68 ± 0.50 | 1672.43 ± 10.00 | 29.86 ± 2.00 |
| 40 wt % HPBS/MVQ | 0.19 ± 0.05 | 0.48 ± 0.10 | 6.47 ± 0.50 | 1064.72 ± 10.00 | 34.60 ± 2.00 |
| Samples | Energy Dissipation a DE/% | ||
|---|---|---|---|
| Strain-100% | Strain-200% | Strain-300% | |
| MVQ | 14.93 | 28.11 | 33.96 |
| 10 wt % LPBS/MVQ | 29.08 | 32.38 | 45.08 |
| 20 wt % LPBS/MVQ | 29.13 | 35.48 | 44.17 |
| 30 wt % LPBS/MVQ | 29.83 | 36.85 | 46.81 |
| 40 wt % LPBS/MVQ | 30.44 | 38.81 | 41.00 |
| 40 wt % PBS/MVQ | 19.22 | 28.78 | 21.27 |
| 40 wt % HPBS/MVQ | 17.63 | 25.98 | 29.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Jiang, N.; Zhang, D.; He, B.; Chen, X. Study on Optimization of Damping Performance and Damping Temperature Range of Silicone Rubber by Polyborosiloxane Gel. Polymers 2020, 12, 1196. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051196
Zhao J, Jiang N, Zhang D, He B, Chen X. Study on Optimization of Damping Performance and Damping Temperature Range of Silicone Rubber by Polyborosiloxane Gel. Polymers. 2020; 12(5):1196. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051196
Chicago/Turabian StyleZhao, Jiang, Nan Jiang, Dongsheng Zhang, Bobing He, and Xian Chen. 2020. "Study on Optimization of Damping Performance and Damping Temperature Range of Silicone Rubber by Polyborosiloxane Gel" Polymers 12, no. 5: 1196. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051196