Different Strategies for the Preparation of Galactose-Functionalized Thermo-Responsive Nanogels with Potential as Smart Drug Delivery Systems
Abstract
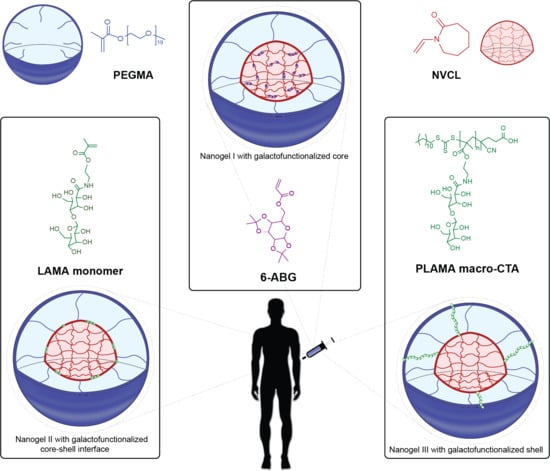
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PLAMA-Macro CTA
2.3. Synthesis of PNVCL:PEGMA Galactosilated Nanogels I and II via SFEP/Free Radical Polymerization
2.4. Synthesis of PNVCL:PEGMA Galactosilated Nanogels III vía SFEP/RAFT Polymerization
2.5. Hydrodynamic Diameter, Sensitivity to Temperature and Zeta Potential
2.6. Molecular Weight, Radius of Gyration, and Second Virial Coefficient
2.7. Chemical Structure and Composition
2.8. Morphological Analyses
3. Results and Discussion
3.1. Synthesis of PLAMA-Macro CTA
3.2. Synthesis of PNVCL:PEGMA Galactosylated Nanogels via SFEP/Free Radical or SFEP/RAFT Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spain, S.G.; Gibson, M.I.; Cameron, N.R. Recent advances in the synthesis of well-defined glycopolymers. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2059–2072. [Google Scholar] [CrossRef]
- Werz, D.B.; Seeberger, P.H. Carbohydrates as the next frontier in pharmaceutical research. Chem. Eur. J. 2005, 11, 3194–31206. [Google Scholar] [CrossRef] [PubMed]
- Voit, B.; Appelhans, D. Glycopolymers of various architectures—more than mimicking nature. Macromol. Chem. Phys. 2010, 211, 727–735. [Google Scholar] [CrossRef]
- Ozyurek, Z.; Franke, K.; Nitschke, M.; Schulze, R.; Simon, F.; Eichhorn, K.J.; Pompe, T.; Werner, C.; Voit, B. Sulfated glyco-block copolymers with specific receptor and growth factor binding to support cell adhesion and proliferation. Biomaterials 2009, 30, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Nitschke, M.; Gramm, S.; Ozuyrek, Z.; Voit, B.; Werner, C.; Muller, A.H. Immobilized hyperbranched glycoacrylate films as bioactive supports. Macromol. Biosci. 2006, 6, 658–666. [Google Scholar] [CrossRef]
- Ogata, M.; Hidari, K.I.P.J.; Kozaki, W.; Murata, T.; Hiratake, J.; Park, E.Y.; Suzuki, T.; Usui, T. Molecular design of spacer-N-linked sialoglycopolypeptide as polymeric inhibitors against influenza virus infection. Biomacromolecules 2009, 10, 1894–1903. [Google Scholar] [CrossRef]
- Miyagawa, A.; Kasuya, M.C.Z.; Hatanaka, K. Inhibitory effects of glycopolymers having globotriose and/or lactose on cytotoxicity of Shiga toxin 1. Carbohydr. Polym. 2007, 67, 260–264. [Google Scholar] [CrossRef]
- Shmidt, T.; Janik, I.; Kadlubowski, S.; Ulanski, P.; Rosiak, J.M.; Reichelt, R.; Arndt, K.F. Pulsed electron beam irradiation of dilute aqueous poly(vinyl methyl ether) solutions. Polymer 2005, 46, 9908–9918. [Google Scholar] [CrossRef]
- Kwon, J.; Drumright, R.; Siegwart, D. The development of micro-gels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar]
- Siirilä, J.; Hietala, S.; Ekholm, F.S.; Tenhu, H. Glucose and maltose surface-functionalized thermoresponsive poly( N-vinylcaprolactam) nanogels. Biomacromolecules 2020, 21, 955–965. [Google Scholar] [CrossRef]
- Schofield, C.L.; Haines, A.H.; Field, R.A.; Russell, D.A. Silver and gold glyconanoparticles for colorimetric bioassays. Langmuir 2006, 22, 6707–6711. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Carbohydrate-carbohydrate interaction as an initial step in cell recognition. Pure Appl. Chem. 1991, 63, 473–482. [Google Scholar] [CrossRef]
- de la Fuente, J.M.; Barrientos, A.G.; Rojas, T.C.; Rojo, J.; Cañada, J.; Fernandez, A.; Penade’s, S. Gold glyconanoparticles as water-soluble polyvalent models to study carbohydrate interactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2257–2261. [Google Scholar] [CrossRef]
- Rojo, J.; Morales, J.C.; Penade’s, S. Carbohydrate-carbohydrate interactions in biological systems. Top. Curr. Chem. 2002, 218, 45–92. [Google Scholar]
- de la Fuente, J.M.; Eaton, P.; Barrientos, A.G.; Menendez, M.; Penade’s, S. Thermodynamic evidence for Ca2+-mediated self-aggregation of Lewis X gold glyconanoparticles. A model for cell adhesion via carbohydrate-carbohydrate interaction. J. Am. Chem. Soc. 2005, 127, 6192–6197. [Google Scholar] [CrossRef]
- Carvalho de Souza, A.; Halkes, K.M.; Meeldijk, J.D.; Verkleij, A.J.; Vliegenhart, J.F.G.; Kamerling, J.P. Gold glyconanoparticles as probes to explore the carbohydrate-mediated self-recognition of marine sponge cells. ChemBioChem 2005, 6, 828–831. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Y.; Li, P.; Zhuang, J.; Bai, F.; Feng, J.; Yang, X.; Li, Y. Carboxylic acid enriched nanospheres of semiconductor nano-rods for cell imaging. Angew. Chem., Int. Ed. 2008, 47, 1054–1057. [Google Scholar] [CrossRef]
- van Kasteren, S.I.; Campbell, S.J.; Serres, S.; Anthony, D.C.; Sibson, N.R.; Davis, B.G. Glyconanoparticles allow pre-symptomatic in vivo imaging of brain disease. Proc. Natl. Acad. Sci. USA 2009, 106, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.M. Glyconanoparticles for biomedical applications. Comb. Chem. High Throughput Screen. 2011, 14, 173–181. [Google Scholar] [CrossRef]
- Ojeda, R.; de Paz, J.L.; Barrientos, A.G.; Martin-Lomas, M.; Penade´s, S. Preparation of multifunctional glyconanoparticles as a platform for potential carbohydrate-based anticancer vaccines. Carbohydr. Res. 2007, 342, 448–449. [Google Scholar] [CrossRef]
- Wang, J.D.; Matyjaszewski, K. Controlled/”living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT Process. Aust. J. Chem. 2005, 58, 379–410. [Google Scholar] [CrossRef]
- Perrier, S.; Takolpuckdee, P. Macromolecular design via reversible addition–fragmentation chain transfer (RAFT)/xanthates (MADIX) polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5347. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living radical polymerization by the RAFT Process—A First Update. Aust. J. Chem. 2006, 59, 669–692. [Google Scholar] [CrossRef]
- Toyoshima, M.; Miura, Y. Preparation of glycopolymer-substituted gold nanoparticles and their molecular recognition. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1412–1421. [Google Scholar] [CrossRef]
- Ting, S.R.S.; Gregory, A.M.; Stenzel, M.H. Polygalactose containing nanocages: The RAFT process for the synthesis of hollow sugar balls. Biomacromolecules 2009, 10, 342–352. [Google Scholar] [CrossRef]
- Dong, C.; Chaikof, E.L. Self-assembled nanostructures of a biomimetic glycopolymer–polypeptide triblock copolymer. Colloid Polym. Sci. 2005, 283, 1366–1370. [Google Scholar] [CrossRef]
- Cameron, N.R.; Spain, S.G.; Kingham, J.A.; Weck, S.; Albertin, L.; Barker, C.A.; Battaglia, G.; Smart, T.; Blanazs, A. Synthesis of well-defined glycopolymers and some studies of their aqueous solution behavior. Faraday Discuss. 2008, 139, 359–368. [Google Scholar] [CrossRef]
- Das, D.; Patra, P.; Ghosh, P.; Rameshbabu, A.P.; Dhara, S.; Pal, S. Dextrin and poly(lactide)-based biocompatible and biodegradable nanogel for cancer targeted delivery of doxorubicin hydrochloride. Polym. Chem. 2016, 7, 2965–2975. [Google Scholar]
- Roy, R.; Tropper, F.D.; Romanowska, A. Custom-designed glycopolymer syntheses by terpolymerizations. J. Chem. Soc. Chem. Commun. 1992, 21, 1611–1613. [Google Scholar] [CrossRef]
- Glinskii, O.V.; Sud, S.; Mossine, V.V.; Mawhinney, T.P.; Antho-ny, D.C.; Glinsky, G.V.; Pienta, K.J.; Glinsky, V.V. Inhibition of prostate cancer bone metastasis by synthetic TF antigen mim-ic/galectin-3 inhibitor lactulose-L-leucine. Neoplasia 2012, 14, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nangia-Makker, P.; Hogan, V.; Honjo, Y.; Baccarini, S.; Tait, L. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 2002, 24, 1854–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inohara, H.; Raz, A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galec-tin-3 functions. Glycoconjugate J. 1994, 11, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.L.; Li, G.; Podar, K.; Hideshima, T.; Neri, P.; He, D.; Mitsiades, N.; Richardson, P.; Chang, Y.; Schindler, J.; et al. A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resitance and multiple myeloma cells. Cancer Res. 2005, 65, 8350–8358. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.D.; Glinskii, O.V.; Mossine, V.V.; Turk, J.R.; Mawhinney, T.P.; Anthony, D.C.; Henry, C.J.; Huxley, V.H.; Glinsky, G.V.; Pienta, K.J.; et al. Galectin-3 as a potential therapeutic target in tumors arising from malignant endo-thelia. Neoplasia 2007, 9, 662–670. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shen, W.; Chang, Y.; Liu, G.; Cao, A.; An, Z. Bio-compatible, Antifouling, and Thermosensitive Core−Shell Nanogels Synthesized by RAFT Aqueous Dispersion Polymerization. Macromolecules 2011, 44, 2524–2530. [Google Scholar]
- Duan, C.; Gao, J.; Zhang, D.; Jia, L.; Liu, Y.; Zheng, D.; Liu, G.; Tian, X.; Wang, F.; Zhang, Q. Galactose-decorated pH-responsive nanogels for hepatoma targeted delivery of oridin. Biomacromolecules 2011, 12, 4335–4343. [Google Scholar] [CrossRef]
- Quan, S.; Wang, Y.; Zhou, A.; Kumar, P.; Narain, R. Galactose-based thermosensitive nanogels for targeted drug delivery of Iodoazomycin Arabinofuranoside (IAZA) for theranostic management of hypoxic hepatocellular carcinoma. Biomacromolecules 2015, 16, 1978–1986. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, C.Y.; Pan, C.Y. Galactose-based amphiphilic block copolymers: Synthesis, micellization, and bioapplication. Biomacromolecules 2013, 14, 1444–1451. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Gonzalez-Ayon, M.A.; Cortez-Lemus, N.A.; Zizumbo- Lopez, A.; Licea-Claverie, A. Nanogels of poly(N-vinylcaprolactam) core and polyethyleneglycol shell by surfactant free emulsion polymerization. Soft Mater. 2014, 12, 315–325. [Google Scholar] [CrossRef]
- Sun, L.; Wei, H.; Zhang, X.; Meng, C.; Kang, G.; Ma, W.; Ma, L.; Wang, B.; Yu, C. Synthesis of polymeric micelles with dual-functional sheddable PEG stealth for enhanced tumor-targeted drug delivery. Polym. Chem. 2020, 11, 4469–4476. [Google Scholar] [CrossRef]
- Gonzalez-Ayon, M.A.; Sañudo-Barajas, J.A.; Picos-Corrales, L.A.; Licea-Claverie, A. PNVCL-PEGMA nanohydrogels with tailored transition temperature for controlled delivery of 5-fluorouracil. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2662–2672. [Google Scholar] [CrossRef]
- Moad, G.; Chong, Y.K.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT polymerization: The synthesis of polymers with defined end-groups. Polymers 2005, 46, 8458–8468. [Google Scholar] [CrossRef]
- Narain, R.; Armes, S.P. Synthesis and aqueous solution properties of novel sugar methacrylate-based homopolymers and block copolymers. Biomacromolecules 2003, 4, 1746–1758. [Google Scholar] [CrossRef]
- Figg, C.A.; Simula, A.; Gebre, K.A.; Tucker, B.S.; Haddleton, D.M.; Sumerlin, B.S. Polymerization-induced thermal self-assembly (PITSA). Chem. Sci. 2015, 6, 1230–1236. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Medina, A.; Cornejo-Bravo, J.M.; Licea-Claverıe, A. Synthesis of pH and temperature sensitive, core–shell nano/microgels, by one pot, soap-free emulsion polymerization. J. Colloid Interface Sci. 2012, 369, 82–90. [Google Scholar] [CrossRef]
- Manzanares-Guevara, L.A.; Angel Licea-Claverie, A.; Paraguay-Delgado, F. Preparation of stimuli responsive nanogels based on poly(N,N-diethylaminoethyl methacrylate) by a simple “surfactant-free” methodology. Soft Mater. 2018, 16, 37–50. [Google Scholar] [CrossRef]
- Lou, S.; Gao, S.; Wang, W.; Zhang, M.; Zhang, J.; Wang, C.; Li, C.; Kong, D.; Zhao, Q. Galactose-functionalized multi-responsive nanogels for hepatoma-targeted drug delivery. Nanoscale 2015, 7, 3137–3146. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Licea-Claverıe, A.; Arndt, K.F. Bisensi-tive core-shell nanohydrogels by e-beam irradiation of micelles. React. Funct. Polym. 2014, 75, 31–40. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Licea-Claverıe, A.; Arndt, K.F. Core–shell nanogels by RAFT crosslinking polymerization: Synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4277–4287. [Google Scholar] [CrossRef]
- Burchard, W. Solution properties of branched macromolecules. Adv. Polym. Sci. 1999, 143, 113–194. [Google Scholar]
- Shah, Z.S.; Pal, A.; Gude, R.; Devi, S. Synthesis and characterization of thermo-responsive copolymeric nanoparticles of poly(methyl methacrylate-co-N-vinylcaprolactam). Eur. Polym. J. 2010, 46, 958–967. [Google Scholar] [CrossRef]
- Sugihara, S.; Kanaoka, S.; Aoshima, S. thermosensitive random copolymers of hydrophilic and hydrophobic monomers obtained by living cationic copolymerization. Macromolecules 2004, 37, 1711–1749. [Google Scholar] [CrossRef]
- Li, X.; Ge, S.; Yang, J.; Chang, R.; Liang, C.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q. Synthesis and study the properties of StNPs/gum nanoparticles for salvianolic acid B-oral delivery system. Food Chem. 2017, 229, 111–119. [Google Scholar] [CrossRef]
- Dai, Q.; Zhu, X.; Abbas, S.; Karangwa, E.; Zhang, X.; Xia, S.; Feng, B.; Jia, C. Stable nanoparticles prepared by heating electrostatic complexes of whey protein isolate-dextran conjugate and chondroitin sulfate. J. Agric. Food Chem. 2015, 63, 4179–4189. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Yao, P.; Jiang, M. Lysozyme-dextran core-shell nanogels prepared via a green process. Langmuir 2008, 24, 3486–3492. [Google Scholar] [CrossRef]
- Hwang, T.L.; Aljuffali, I.A.; Lin, C.F.; Chang, Y.T.; Fang, J.Y. Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: Lipid nanoparticles versus polymeric nanoparticles. Int. J. Nanomed. 2015, 10, 371–385. [Google Scholar]








| Nanogel | Rh a (nm) | PDI a | Mw b (g mol−1) | Rg b (nm) | A2 b (mol mL g−2) | ρ-Parameter c |
|---|---|---|---|---|---|---|
| Nanogels I | ||||||
| N46 | 185 | 0.14 | 2.20 × 1010 | 210 | 3.75 × 10−7 | 1.13 |
| N45 | 120 | 0.11 | 1.57 × 109 | 173 | 2.01 × 10−9 | 1.44 |
| N48 | 61 | 0.08 | 8.74 × 108 | 76 | 4.83 × 10−7 | 1.24 |
| Nanogels II | ||||||
| N32 | 145 | 0.37 | 1.11 × 108 | 143 | 2.64 × 10−6 | 0.98 |
| N50 | 56 | 0.19 | 3.65 × 106 | 44 | 4.12 × 10−6 | 0.78 |
| N51 | 45 | 0.18 | 3.53 × 106 | 43 | 4.01 × 10−6 | 0.95 |
| Nanogels III | ||||||
| N42 | 144 | 0.33 | 9.10 × 106 | 112 | 1.18 × 10−4 | 0.78 |
| N44 | 156 | 0.18 | 7.90 × 107 | 154 | 2.50 × 10−6 | 0.98 |
| Nanogel | Feed Composition (mol%) a | Product Composition (mol%) a | ||||
|---|---|---|---|---|---|---|
| NVCL | PEGMA | GAL | NVCL | PEGMA | GAL | |
| Nanogels I | ||||||
| N46 | 66.7 | 5.5 | 27.8 | 65.5 | 6.5 | 28.1 |
| N45 | 76.9 | 6.3 | 16.8 | 65.3 | 7.6 | 27.2 |
| N48 | 83.9 | 6.9 | 9.2 | 59.6 | 16.6 | 23.8 |
| Nanogels II | ||||||
| N32 | 92.2 | 7.8 | 0 | 92 | 8 | 0 |
| N50 | 80.3 | 7.8 | 11.9 | 65.4 | 26.9 | 7.7 |
| N51 | 85.4 | 8.3 | 6.3 | 67.9 | 26.4 | 5.7 |
| Nanogels III | ||||||
| N42 | 86.0 | 10.3 | 3.7 | 56.6 | 24.5 | 18.9 |
| N44 | 88.3 | 8.4 | 3.3 | 76 | 12 | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ayón, M.A.; Licea-Claverie, A.; Sañudo-Barajas, J.A. Different Strategies for the Preparation of Galactose-Functionalized Thermo-Responsive Nanogels with Potential as Smart Drug Delivery Systems. Polymers 2020, 12, 2150. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092150
González-Ayón MA, Licea-Claverie A, Sañudo-Barajas JA. Different Strategies for the Preparation of Galactose-Functionalized Thermo-Responsive Nanogels with Potential as Smart Drug Delivery Systems. Polymers. 2020; 12(9):2150. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092150
Chicago/Turabian StyleGonzález-Ayón, Mirian A., Angel Licea-Claverie, and J. Adriana Sañudo-Barajas. 2020. "Different Strategies for the Preparation of Galactose-Functionalized Thermo-Responsive Nanogels with Potential as Smart Drug Delivery Systems" Polymers 12, no. 9: 2150. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092150