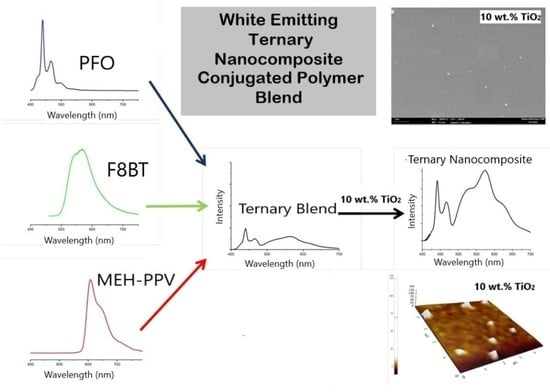
Improving Photophysical Properties of White Emitting Ternary Conjugated Polymer Blend Thin Film via Additions of TiO2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation and Characterizations
3. Results and Discussion
3.1. Binary System PFO/MEH-PPV
3.2. PFO/F8BT/MEH-PPV Ternary System
3.2.1. Effect of F8BT Ratio
3.2.2. Effect of TiO2 Nanoparticles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duarte, L.G.T.; Germino, J.C.; Mendes, R.A.; Berbigier, J.F.; Faleiros, M.M.; Rodembusch, F.S.; Atvars, T.D.Z. The role of a simple and effective salicylidene derivative. Spectral broadening and performance improvement of PFO-based all-solution processed OLEDs. Dye. Pigment. 2019, 171, 107671. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, P.-I.; Ong, G.L.; Tan, S.H.; Tan, Z.W.; Hii, Y.H.; Wong, Y.L.; Cheah, K.S.; Yap, S.L.; Ong, T.S.; et al. Photophysical and Electroluminescence Characteristics of Polyfluorene Derivatives with Triphenylamine. Polymers 2019, 11, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, S.-H.; Jou, J.-H.; Chavhan, S.D.; Su, T.-H.; Yuan, C.-H.; Wen, J.W.; Chen, P.-R.; Tung, F.-C.; Tsai, Y.-C. High efficiency color-temperature tunable organic light-emitting diode. J. Mater. Chem. C 2019, 7, 15322–15334. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.-Y.; Jiang, Z.-Q.; Liao, L.-S. High-Quality White Organic Light-Emitting Diodes Composed of Binary Emitters with Color Rendering Index Exceeding 80 by Utilizing Color Remedy Strategy. Adv. Funct. Mater. 2019, 29, 1807541. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Shiu, Y.-J.; Wu, Y.-J.; Huang, W.-Y. Simple structured color tunable white organic light-emitting diodes utilizing an ambipolar anthracene derivative with low-lying LUMO. Org. Electron. 2020, 76, 105454. [Google Scholar] [CrossRef]
- Huang, J.; Li, G.; Wu, E.; Xu, Q.; Yang, Y. Achieving High-Efficiency Polymer White-Light-Emitting Devices. Adv. Mater. 2006, 18, 114–117. [Google Scholar] [CrossRef]
- Huang, T.-H.; Chi, X.-C.; Xu, T.-N.; Zhang, J.-R.; Xu, H.-Y.; Zhu, Z.-Y.; Yu, R.-B.; Wang, Y.-H.; Zhang, H.-Z. Effect of Ag nanoparticles on the photoluminescence of poly[2 -methoxy-5-(2-ethylhexyloxy)-1,4-phenylene-vinylene]. J. Photochem. Photobiol. A Chem. 2018, 356, 334–339. [Google Scholar] [CrossRef]
- Al-Asbahi, B.; Qaid, S.M.H.; Jumali, M.H.H.; AlSalhi, M.S.; Aldwayyan, A.S. Long-range dipole–dipole energy transfer enhancement via addition of SiO 2/TiO 2 nanocomposite in PFO/MEH-PPV hybrid thin films. J. Appl. Polym. Sci. 2019, 136, 47845. [Google Scholar] [CrossRef]
- Kandulna, R.; Choudhary, R.B.; Singh, R. TiO2 reinforced PMMA-TiO2 nanocomposite for its application in organic light emitting diode (OLED) as electron transport layer material. Dae Solid State Phys. Symp. 2017 2018, 1942, 110057. [Google Scholar] [CrossRef]
- Fuzi, S.A.A.; Jumali, M.H.H.; Al-Asbahi, B.A.; Qaid, S.M. Photophysical and energy transfer mechanism studies of Poly (9,9-di-n-octylflourenyl-2,7-diyl)/Fluorol 7GA/Poly [2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] ternary organic blend films. Thin Solid Films 2019, 683, 90–96. [Google Scholar] [CrossRef]
- Morgado, J.; Friend, R.H.; Cacialli, F. Improved efficiency of light-emitting diodes based on polyfluorene blends upon insertion of a poly(p-phenylene vinylene) electron- confinement layer. Appl. Phys. Lett. 2002, 80, 2436–2438. [Google Scholar] [CrossRef]
- Heydari, E.; Pastoriza-Santos, I.; Flehr, R.; Liz-Marzán, L.M.; Stumpe, J. Nanoplasmonic Enhancement of the Emission of Semiconductor Polymer Composites. J. Phys. Chem. C 2013, 117, 16577–16583. [Google Scholar] [CrossRef]
- Stevenson, R.; Riehn, R.; Milner, R.G.; Richards, D.; Moons, E.; Kang, D.J.; Blamire, M.G.; Morgado, J.; Cacialli, F. Ultraviolet–visible near-field microscopy of phase-separated blends of polyfluorene-based conjugated semiconductors. Appl. Phys. Lett. 2001, 79, 833–835. [Google Scholar] [CrossRef]
- Wang, X.; Groff, L.C.; McNeill, J.D. Multiple Energy Transfer Dynamics in Blended Conjugated Polymer Nanoparticles. J. Phys. Chem. C 2014, 118, 25731–25739. [Google Scholar] [CrossRef]
- Nedumpara, R.J.; Manu, P.; Vallabhan, C.; Nampoori, V.; Radhakrishnan, P. Energy transfer studies in dye mixtures in different solvent environments. Opt. Laser Technol. 2008, 40, 953–957. [Google Scholar] [CrossRef]
- Dos Santos, M.C.; Algar, W.R.; Medintz, I.L.; Hildebrandt, N. Quantum dots for Förster Resonance Energy Transfer (FRET). TrAC Trends Anal. Chem. 2020, 125, 115819. [Google Scholar] [CrossRef]
- Thomas, S.; Grohens, Y.; Jyotishkumar, P. (Eds.) Characterization of Polymer Blends: Miscibility, Morphology and Interfaces, 1st ed.; Wiley-VCH: Weinheim, Germany, 2015; ISBN 978-3-527-33153-6. [Google Scholar]
- Lee, T.-W.; Park, J.H.; Park, O.O.; Lee, J.-H.; Kim, Y.C. A systematic doping strategy to control the emission spectrum of ternary luminescent polymer blends for white emission. Opt. Mater. 2007, 30, 486–491. [Google Scholar] [CrossRef]
- Al-Asbahi, B.; Jumali, M.H.; Yap, C.C.; Salleh, M.M.; AlSalhi, M. Inhibition of dark quenching by TiO2 nanoparticles content in novel PFO/Fluorol 7GA hybrid: A new role to improve OLED performance. Chem. Phys. Lett. 2013, 570, 109–112. [Google Scholar] [CrossRef]
- Al-Asbahi, B. Influence of SiO2/TiO2 Nanocomposite on the Optoelectronic Properties of PFO/MEH-PPV-Based OLED Devices. Polymers 2018, 10, 800. [Google Scholar] [CrossRef] [Green Version]
- Jokinen, K.; Bykov, A.; Sliž, R.; Remes, K.; Fabritius, T.; Myllylä, R. Luminescence and spectrum variations caused by thermal annealing in undoped and doped polyfluorene OLEDs. Solid-State Electron. 2015, 103, 184–189. [Google Scholar] [CrossRef]
- Winfield, J.M.; Van Vooren, A.; Park, M.-J.; Hwang, D.-H.; Cornil, J.; Kim, J.-S.; Friend, R.H. Charge-transfer character of excitons in poly[2,7-(9,9-di-n-octylfluorene)(1−x)-co-4,7-(2,1,3-benzothiadiazole)(x)]. J. Chem. Phys. 2009, 131, 035104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananthakrishnan, N.; Padmanaban, G.; Ramakrishnan, S.; Reynolds, J.R. Tuning Polymer Light-Emitting Device Emission Colors in Ternary Blends Composed of Conjugated and Nonconjugated Polymers. Macromolecules 2005, 38, 7660–7669. [Google Scholar] [CrossRef]
- Arango, A.C.; Carter, S.A.; Brock, P.J. Charge transfer in photovoltaics consisting of interpenetrating networks of conjugated polymer and TiO[sub 2] nanoparticles. Appl. Phys. Lett. 1999, 74, 1698–1700. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Bai, Z.; Liu, B.; Li, T.; Lu, D. From Starting Formation to the Saturation Content of the β-Phase in Poly(9,9-dioctylfluorene) Toluene Solutions. J. Phys. Chem. C 2017, 121, 14443–14450. [Google Scholar] [CrossRef]
- Perevedentsev, A.; Chander, N.; Kim, J.; Bradley, D.D.C. Spectroscopic properties of poly(9,9-dioctylfluorene) thin films possessing varied fractions of β-phase chain segments: Enhanced photoluminescence efficiency via conformation structuring. J. Polym. Sci. Part. B Polym. Phys. 2016, 54, 1995–2006. [Google Scholar] [CrossRef] [Green Version]
- Aharon, E.; Albo, A.; Kalina, M.; Frey, G.L. Stable Blue Emission from a Polyfluorene/Layered-Compound Guest/Host Nanocomposite. Adv. Funct. Mater. 2006, 16, 980–986. [Google Scholar] [CrossRef]
- Voigt, M.; Chappell, J.; Rowson, T.; Cadby, A.J.; Geoghegan, M.; Jones, R.A.L.; Lidzey, D.G. The interplay between the optical and electronic properties of light-emitting-diode applicable conjugated polymer blends and their phase-separated morphology. Org. Electron. 2005, 6, 35–45. [Google Scholar] [CrossRef]
- Soman, A.; Sajeev, A.K.; Rajeev, K.; Narayanan, U.K.N. Reversible Shift from Excitonic to Excimer Emission in Fluorescent Organic Light-Emitting Diodes: Dependence on Deposition Parameters and Electrical Bias. ACS Omega 2020, 5, 1698–1707. [Google Scholar] [CrossRef] [Green Version]
- Müllen, K.; Scherf, U. (Eds.) Organic Light Emitting Devices: Synthesis, Properties and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2005; ISBN 978-3-527-60723-5. [Google Scholar]
- Han, Z.; Zhang, J.; Yang, X.; Zhu, H.; Cao, W. Synthesis and application in solar cell of poly(3-octylthiophene)/titania nanotubes composite. Org. Electron. 2010, 11, 1449–1460. [Google Scholar] [CrossRef]
- Chen, G.; Wang, P. Alteration of the optical properties of poly 9,9′-dioctylfluorene by TiO2 nanocrystalline. J. Non-Crystalline Solids 2006, 352, 2536–2538. [Google Scholar] [CrossRef]
- Gaur, M.S.; Singh, P.K.; Suruchi; Chauhan, R.S. Structural and thermal properties of polysulfone-ZnO nanocomposites. J. Therm. Anal. Calorim. 2012, 111, 743–751. [Google Scholar] [CrossRef]
- Wang, K.H.; Choi, M.H.; Koo, C.M.; Xu, M.; Chung, I.J.; Jang, M.C.; Choi, S.W.; Song, H.H. Morphology and physical properties of polyethylene/silicate nanocomposite prepared by melt intercalation. J. Polym. Sci. Part. B Polym. Phys. 2002, 40, 1454–1463. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Yu, F.-X.; Dong, Y.; Jia, Y.-L.; Ma, X.-J.; Xu, Q.; Deng, Y.; Xiong, Z.; Gao, C.-H. An efficient CsPbBr3 perovskite light-emitting diode by employing 1,3,5-tri(m-pyrid-3-yl-phenyl)benzene as a hole and exciton blocking layer. J. Lumin. 2020, 219, 116915. [Google Scholar] [CrossRef]
- Jumali, M.H.H.; Al-Asbahi, B.A.; Yap, C.C.; Salleh, M.M.; Alsalhi, M.S. Optoelectronic property enhancement of conjugated polymer in poly (9, 9′-di-n-octylfluorenyl-2.7-diyl)/titania nanocomposites. Thin Solid Films 2012, 524, 257–262. [Google Scholar] [CrossRef]
- Higgins, A.M.; Cadby, A.J.; Lidzey, D.G.; Dalgliesh, R.M.; Geoghegan, M.; Jones, R.A.L.; Martin, S.; Heriot, S.Y. The Impact of Interfacial Mixing on Förster Transfer at Conjugated Polymer Heterojunctions. Adv. Funct. Mater. 2009, 19, 157–163. [Google Scholar] [CrossRef]
- Al-Asbahi, B.; Jumali, M.H.H.; AlSalhi, M. Enhanced Optoelectronic Properties of PFO/Fluorol 7GA Hybrid Light Emitting Diodes via Additions of TiO2 Nanoparticles. Polymers 2016, 8, 334. [Google Scholar] [CrossRef]
- Bajpai, M.; Srivastava, R.; Kamalasanan, M.; Tiwari, R.; Chand, S. Charge transport and microstructure in PFO:MEH-PPV polymer blend thin films. Synth. Met. 2010, 160, 1740–1744. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, H.; Cao, F.; Wang, S.; Wu, J.; Dou, Y.; Zhang, J.; Chen, J.; Zhao, D.; Yang, X. Efficient All-Solution-Processed Perovskite Light-Emitting Diodes Enabled by Small-Molecule Doped Electron Injection Layers. Adv. Opt. Mater. 2019, 8. [Google Scholar] [CrossRef]









| Roughness | Binary | Ternary | Nanocomposite (10 wt.%) | Nanocomposite (20 wt.%) | Nanocomposite (30 wt.%) |
|---|---|---|---|---|---|
| Rq (nm) | 1.959 | 2.129 | 2.373 | 8.990 | 10.407 |
| Ra (nm) | 1.352 | 1.424 | 1.612 | 3.184 | 5.106 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Bati, S.; Hj. Jumali, M.H.; Al-Asbahi, B.A.; Ibtehaj, K.; Yap, C.C.; Qaid, S.M.H.; Ghaithan, H.M.; Farooq, W.A. Improving Photophysical Properties of White Emitting Ternary Conjugated Polymer Blend Thin Film via Additions of TiO2 Nanoparticles. Polymers 2020, 12, 2154. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092154
Al-Bati S, Hj. Jumali MH, Al-Asbahi BA, Ibtehaj K, Yap CC, Qaid SMH, Ghaithan HM, Farooq WA. Improving Photophysical Properties of White Emitting Ternary Conjugated Polymer Blend Thin Film via Additions of TiO2 Nanoparticles. Polymers. 2020; 12(9):2154. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092154
Chicago/Turabian StyleAl-Bati, Sameer, Mohammad Hafizuddin Hj. Jumali, Bandar Ali Al-Asbahi, Khatatbeh Ibtehaj, Chi Chin Yap, Saif M. H. Qaid, Hamid M. Ghaithan, and W. A. Farooq. 2020. "Improving Photophysical Properties of White Emitting Ternary Conjugated Polymer Blend Thin Film via Additions of TiO2 Nanoparticles" Polymers 12, no. 9: 2154. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092154