Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review
Abstract
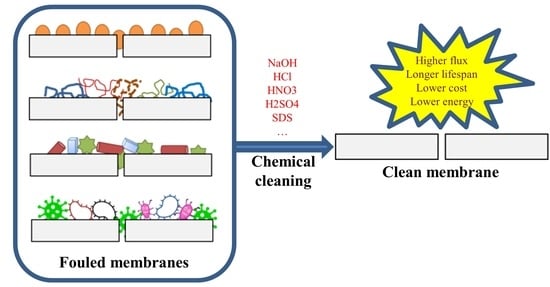
:1. Introduction
2. Membrane Foulants and Affecting Factors of Fouling
2.1. Foulants and Mechanism of Fouling in Microfiltration Membrane
2.2. Affecting Factors of Membrane Fouling
2.2.1. Membrane Properties Effect on Membrane Fouling
2.2.2. Solution Properties Effect on Membrane Fouling
2.2.3. Operational Conditions Effect on Membrane Fouling
3. Microfiltration Membrane Cleaning Methods
3.1. Physical Cleaning of Membranes
3.1.1. Forward and Reverse Flushing
3.1.2. Air Flushing
3.1.3. Backwashing
3.1.4. Relaxation
3.1.5. Sponge Ball
3.2. Chemical Cleaning
3.2.1. Chemical Cleaning Process
- Fouled membranes immersed in chemicals, such as “clean-in-place” (CIP),
- Soaking of fouled membranes using high-concentration cleaning agents using separate tanks, such as “clean-out-off-place” (COP),
- Adding chemicals to the feed stream, such as “chemical wash” (CW),
- Combining physical and chemical cleaning, such as “chemical enhanced backwash” (CEB).
- Bulk reactions for cleaning reagents,
- Transport of chemical agent to the membrane interface,
- Transport of chemical agent into the foulant layer,
- Cleaning reactions in the fouling layer,
- Transport of cleaning reaction products back to the interface,
- Transport of product to the bulk solution.
3.2.2. Performance of Cleaning Agents in Microfiltration
- The removal of foulants is possible,
- The morphology of foulants may change (swelling, compaction), and/or
- The surface chemistry of foulants may be changed by using hydrophobicity or an electrical charge.
4. Parameters Affecting Cleaning Efficiency
4.1. Cleaning Time
4.2. Temperature of the Cleaning Process
- By changing the chemical reaction balance,
- By changing the solubility of fouling materials and/or reaction products during the cleaning, and
- By changing the reaction kinetics.
4.3. Concentration of Chemicals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef] [Green Version]
- Nageeb, M. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. Org. Pollut. Monit. Risk Treat. 2013, 30, 167–194. [Google Scholar] [CrossRef] [Green Version]
- Comninellis, C.; Kapalka, A.; Malato, S.A.; Parsons, S.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar] [CrossRef]
- Petrovic, M.; Radjenovic, J.; Barcelo, D. Advanced oxidation processes (AOPs) applied for wastewater and drinking water treatment. Elimination of pharmaceuticals. Holist. Approach Environ. 2011, 1, 63–74. [Google Scholar]
- Andreozzi, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Ferro, S. Electrochemical oxidation of organic pollutants for the wastewater treatment: Direct and indirect processes. Chem. Soc. Rev. 2006, 35, 1324–1340. [Google Scholar] [CrossRef]
- Bauer, R.; Fallmann, H. The Photo-Fenton Oxidation—A cheap and efficient wastewater treatment method. Res. Chem. Intermed. 1997, 23, 341–354. [Google Scholar] [CrossRef]
- Friedmann, D.; Mendive, C.B.; Bahnemann, D.W. TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B Environ. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Vidal, A. Developments in solar photocatalysis for water purification. Chemosphere 1998, 36, 2593–2606. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Bouwer, E.J.; Crowe, P.B. Biological Processes in Drinking Water Treatment. J. Am. Water Work. Assoc. 1988, 80, 82–93. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Chaplin, B.P. Advantages, Disadvantages, and Future Challenges of the Use of Electrochemical Technologies for Water and Wastewater Treatment; Elsevier BV: Oxford, UK, 2018; pp. 451–494. [Google Scholar]
- Amuda, O.S.; Amoo, I.A. Coagulation/flocculation process and sludge conditioning in beverage industrial wastewater treatment. J. Hazard. Mater. 2007, 141, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Tzoupanos, N.D.; Zouboulis, A.I. Coagulation-flocculation processes in water/wastewater treatment: The application of new generation of chemical reagents. In Proceedings of the 6th IASME/WSEAS Int. Conf. on Heat Transfer, Thermal Engineering And Environment (HTE 8), Rhodes Island, Greece, 20–22 August 2009; pp. 309–317. [Google Scholar]
- Madaeni, S.S.; Yeganeh, M.K. Microfiltration of Emulsified Oil Wastewater. J. Porous Mater. 2003, 10, 131–138. [Google Scholar] [CrossRef]
- El-Ghaffar, M.A.A.; Tieama, H.A. A Review of Membranes Classifications, Configurations, Surface Modifications, Characteristics and Its Applications in Water Purification. Chem. Biomol. Eng. 2017, 2, 57–82. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A Review on Membrane Technology and Chemical Surface Modification for the Oily Wastewater Treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef] [PubMed]
- Zirehpour, A. Rahimpour, A. Membranes for wastewater treatment. In Nanostructured Polymer Membranes; John Wiley & Sons Ltd.: London, UK, 2016; Volume 2, pp. 159–207. [Google Scholar]
- Baker, R.W. Membrane technology and applications. In Membrane Technology and Applications; John Wiley & Sons Ltd.: London, UK, 2004; pp. 275–299. [Google Scholar]
- Grant, R. Membrane Separations. Mater. Manuf. Process. 1989, 4, 483–503. [Google Scholar] [CrossRef]
- Urošević, T.; Povrenović, D.; Vukosavljević, P.; Urošević, I.; Stevanović, S. Recent developments in microfiltration and ultrafiltration of fruit juices. Food Bioprod. Process. 2017, 106, 147–161. [Google Scholar] [CrossRef]
- Li, P.; Zhang, S.; Lv, Y.; Ma, G.; Zuo, X. Fouling mechanism and control strategy of inorganic membrane. E3S Web Conf. 2020, 194, 04047. [Google Scholar] [CrossRef]
- Huang, S.; Ras, R.H.; Tian, X. Antifouling membranes for oily wastewater treatment: Interplay between wetting and membrane fouling. Curr. Opin. Colloid Interface Sci. 2018, 36, 90–109. [Google Scholar] [CrossRef] [Green Version]
- Devanadera, M.; Dalida, M. Fouling of Ceramic Microfiltration Membrane by Soluble Algal Organic Matter (Saom) from Chlorella Sp. and Aeruginosa M. and its Mitigation Using Feed-Pretreatment. In Proceedings of the 14 th International Conference on Environmental Science and Technology, Rhodes, Greece, 3–5 September 2015. [Google Scholar]
- Sioutopoulos, D.; Karabelas, A.; Mappas, V. Membrane Fouling Due to Protein—Polysaccharide Mixtures in Dead-End Ultrafiltration; the Effect of Permeation Flux on Fouling Resistance. Membranes 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickhout, J.; Moreno, J.; Biesheuvel, P.; Boels, L.; Lammertink, R.; de Vos, W. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Bazin, M.M.; Nakamura, Y.; Ahmad, N. Chemical Cleaning of Microfiltration Ceramic Membrane Fouled by Nom. J. Teknol. 2018, 80, 95–103. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. The effect of permeate flux on membrane fouling during microfiltration of oily water. J. Membr. Sci. 2017, 525, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, K.L.; Bram, M.V.; Pedersen, S.; Yang, Z. Membrane Fouling for Produced Water Treatment: A Review Study from a Process Control Perspective. Water 2018, 10, 847. [Google Scholar] [CrossRef] [Green Version]
- France, T.C.; Bot, F.; Kelly, A.L.; Crowley, S.V.; O’Mahony, J.A. The influence of temperature on filtration performance and fouling during cold microfiltration of skim milk. Sep. Purif. Technol. 2021, 262, 118256. [Google Scholar] [CrossRef]
- Kujawa, J.; Chrzanowska, E.; Kujawski, W. Transport properties and fouling issues of membranes utilized for the concentration of dairy products by air-gap membrane distillation and microfiltration. Chem. Pap. 2018, 73, 565–582. [Google Scholar] [CrossRef] [Green Version]
- Garmsiri, E.; Rasouli, Y.; Abbasi, M.; Izadpanah, A.A. Chemical cleaning of mullite ceramic microfiltration membranes which are fouled during oily wastewater treatment. J. Water Process. Eng. 2017, 19, 81–95. [Google Scholar] [CrossRef]
- Kimura, K.; Honoki, D.; Sato, T. Effective physical cleaning and adequate membrane flux for direct membrane filtration (DMF) of municipal wastewater: Up-concentration of organic matter for efficient energy recovery. Sep. Purif. Technol. 2017, 181, 37–43. [Google Scholar] [CrossRef]
- Shahbazi, Z.; Mirsaeedghazi, H.; Paghaleh, A.S. Selection of the most effective chemical cleaning procedure in the membrane clarification of pomegranate juice. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Bazan, M.; Carpintero-Tepole, V.; La Fuente, E.B.-D.; Drioli, E.; Ascanio, G. On the use of ultrasonic dental scaler tips as cleaning technique of microfiltration ceramic membranes. Ultrasonics 2020, 101, 106035. [Google Scholar] [CrossRef]
- Lam, Z.; Anlauf, H.; Nirschl, H. High-Pressure Jet Cleaning of Polymeric Microfiltration Membranes. Chem. Eng. Technol. 2019, 43, 457–464. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, S.; Zhu, Y.; Shen, Y.; Gao, X.; Shi, W.; Tay, J.H. Efficiencies and mechanisms of the chemical cleaning of fouled polytetrafluoroethylene (PTFE) membranes during the microfiltration of alkali/surfactant/polymer flooding oilfield wastewater. RSC Adv. 2019, 9, 36940–36950. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kang, J.-S.; Kim, H.; Lee, S. Salt cleaning of EfOM-fouled MF membrane for wastewater reclamation. Desalination Water Treat. 2020, 180, 55–66. [Google Scholar] [CrossRef]
- Holman, S.R.; Ohlinger, K.N. An Evaluation of Fouling Potential and Methods to Control Fouling in Microfiltration Membranes for Secondary Wastewater Effluent. Proc. Water Environ. Fed. 2007, 2007, 6417–6444. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, V. Membrane Fouling and Cleaning in Food and Bioprocessing; Elsevier BV: Oxford, UK, 2010; pp. 213–254. [Google Scholar]
- Ulbricht, M.; Ansorge, W.; Danielzik, I.; König, M.; Schuster, O. Fouling in microfiltration of wine: The influence of the membrane polymer on adsorption of polyphenols and polysaccharides. Sep. Purif. Technol. 2009, 68, 335–342. [Google Scholar] [CrossRef]
- Paugam, L.; Delaunay, D.; Diagne, N.W.; Rabiller-Baudry, M. Cleaning of skim milk PES ultrafiltration membrane: On the real effect of nitric acid step. J. Membr. Sci. 2013, 428, 275–280. [Google Scholar] [CrossRef]
- Maskooki, A.; Kobayashi, T.; Mortazavi, S.A.; Maskooki, A. Effect of low frequencies and mixed wave of ultrasound and EDTA on flux recovery and cleaning of microfiltration membranes. Sep. Purif. Technol. 2008, 59, 67–73. [Google Scholar] [CrossRef]
- Kelly, S.T.; Zydney, A.L. Mechanisms for BSA fouling during microfiltration. J. Membr. Sci. 1995, 107, 115–127. [Google Scholar] [CrossRef]
- Liu, C.; Caothien, S.; Hayes, J.; Caothuy, T.; Otoyo, T.; Ogawa, T. Membrane Chemical Cleaning: From Art to Science. In Proceedings of the AWWA Membrane Technology Conference, San Antonio, TX, USA, 4–7 March 2001; 7 March 2001. [Google Scholar]
- Koonani, H.; Amirinejad, M. Combined three mechanisms models for membrane fouling during microfiltration. J. Membr. Sci. Res. 2019, 5, 274–282. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, B.-Q.; Zhou, X.; He, Y.; Hong, H.; Lin, H.; Chen, J. Effects of hydrophilicity/hydrophobicity of membrane on membrane fouling in a submerged membrane bioreactor. Bioresour. Technol. 2015, 175, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Doan, H.; Lohi, A. Fouling in Membrane Filtration and Remediation Methods. Mass Transf. Adv. Sustain. Energy Environ. Oriented Numer. Modeling 2013, 195. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Ismail, A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Salahi, A.; Abbasi, M.; Mohammadi, T. Permeate flux decline during UF of oily wastewater: Experimental and modeling. Desalination 2010, 251, 153–160. [Google Scholar] [CrossRef]
- Ren, L.; Yu, S.; Li, J.; Li, L. Pilot study on the effects of operating parameters on membrane fouling during ultrafiltration of alkali/surfactant/polymer flooding wastewater: Optimization and modeling. RSC Adv. 2019, 9, 11111–11122. [Google Scholar] [CrossRef] [Green Version]
- Thomassen, J.; Faraday, D.; Underwood, B.; Cleaver, J. The effect of varying transmembrane pressure and crossflow velocity on the microfiltration fouling of a model beer. Sep. Purif. Technol. 2005, 41, 91–100. [Google Scholar] [CrossRef]
- Gan, Q.; Howell, J.; Field, R.; England, R.N.; Bird, M.; O’Shaughnessy, C.; MeKechinie, M. Beer clarification by microfiltration—Product quality control and fractionation of particles and macromolecules. J. Membr. Sci. 2001, 194, 185–196. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Mohamamdi, T.; Moghadam, M.K. Chemical cleaning of reverse osmosis membranes. Desalination 2001, 134, 77–82. [Google Scholar] [CrossRef]
- Siembida-Lösch, B. Physical Cleaning. In Encyclopedia of Membranes; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–4. [Google Scholar]
- Madaeni, S.S.; Sharifnia, S.; Moradi, G. Chemical Cleaning of Microfiltration Membranes Fouled by Whey. J. Chin. Chem. Soc. 2001, 48, 179–191. [Google Scholar] [CrossRef]
- Ebrahim, S. Cleaning and regeneration of membranes in desalination and wastewater applications: State-of-the-art. Desalination 1994, 96, 225–238. [Google Scholar] [CrossRef]
- Arnal, J.M.; García-Fayos, B.; Sancho, M. Chapter 3: Membrane cleaning. In Expanding Issues in Desalination; IntechOpen: London, UK, 2011; pp. 63–84. [Google Scholar]
- Chua, H.; Arnot, T.; Howell, J. Controlling fouling in membrane bioreactors operated with a variable throughput. Desalination 2002, 149, 225–229. [Google Scholar] [CrossRef]
- Judd, S.; Judd, C. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: London, UK, 2011. [Google Scholar]
- Lin, J.C.-T.; Lee, D.-J.; Huang, C. Membrane Fouling Mitigation: Membrane Cleaning. Sep. Sci. Technol. 2010, 45, 858–872. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Organic Fouling and Chemical Cleaning of Nanofiltration Membranes: Measurements and Mechanisms. Environ. Sci. Technol. 2004, 38, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Bird, M.; Bartlett, M. Measuring and modelling flux recovery during the chemical cleaning of MF membranes for the processing of whey protein concentrate. J. Food Eng. 2002, 53, 143–152. [Google Scholar] [CrossRef]
- Kubota, Y.M.N.; Hashimoto, T. Part I membranes and applications in water and wastewater. In Advanced Membrane Technology and Applications; Norman, A.G.F., Li, N., Ho, T.M.W.S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 101–165. [Google Scholar]
- Hoornaert, P. Membrane cleaning. Reverse Osmosis 1984, 71, 111–117. [Google Scholar] [CrossRef]
- Al-Obeidani, S.; Al-Hinai, H.; Goosen, M.; Sablani, S.; Taniguchi, Y.; Okamura, H. Chemical cleaning of oil contaminated polyethylene hollow fiber microfiltration membranes. J. Membr. Sci. 2008, 307, 299–308. [Google Scholar] [CrossRef]
- Blanpain-Avet, P.; Migdal, J.; Bénézech, T. Chemical cleaning of a tubular ceramic microfiltration membrane fouled with a whey protein concentrate suspension—Characterization of hydraulic and chemical cleanliness. J. Membr. Sci. 2009, 337, 153–174. [Google Scholar] [CrossRef]
- Makardij, A.; Chen, X.; Farid, M. Microfiltration and Ultrafiltration of Milk. Food Bioprod. Process. 1999, 77, 107–113. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Tavakolian, H.R.; Rahimpour, F. Cleaning Optimization of Microfiltration Membrane Employed for Milk Sterilization. Sep. Sci. Technol. 2011, 46, 571–580. [Google Scholar] [CrossRef]
- Hou, L.; Gao, K.; Li, P.; Zhang, X.; Wang, Z.; Song, P.; Yao, W. A kinetic model for calculating total membrane fouling resistance in chemical cleaning process. Chem. Eng. Res. Des. 2017, 128, 59–72. [Google Scholar] [CrossRef]
- Lee, J.-W.; Jung, J.; Cho, Y.H.; Yadav, S.K.; Baek, K.Y.; Park, H.B.; Hong, S.M.; Koo, C.M. Fouling-Tolerant Nanofibrous Polymer Membranes for Water Treatment. ACS Appl. Mater. Interfaces 2014, 6, 14600–14607. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.T.M.Q.; Howellb, J.A.; Fieldb, R.W.; Englandb, R.; Birdb, M.R. Synergetic cleaning procedure for a ceramic membrane fouled by beer micro®ltration. J. Membr. Sci. 1999, 155, 277–289. [Google Scholar] [CrossRef]
- Woo, Y.C.; Lee, J.K.; Kim, H.-S. Fouling characteristics of microfiltration membranes by organic and inorganic matter and evaluation of flux recovery by chemical cleaning. Desalination Water Treat. 2014, 52, 6920–6929. [Google Scholar] [CrossRef]
- Kweon, J.H.; Jung, J.H.; Lee, S.R.; Hur, H.W.; Shin, Y.; Choi, Y.H. Effects of consecutive chemical cleaning on membrane performance and surface properties of microfiltration. Desalination 2012, 286, 324–331. [Google Scholar] [CrossRef]
- Puspitasari, V.; Granville, A.; Le-Clech, P.; Chen, V. Cleaning and ageing effect of sodium hypochlorite on polyvinylidene fluoride (PVDF) membrane. Sep. Purif. Technol. 2010, 72, 301–308. [Google Scholar] [CrossRef]
- Kang, S.-K.; Choo, K.-H. Use of submerged microfiltration membranes for glass industry wastewater reclamation: Pilot-scale testing and membrane cleaning. Desalination 2006, 189, 170–180. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yasin, N.M.; Derek, C.; Lim, J. Chemical cleaning of a cross-flow microfiltration membrane fouled by microalgal biomass. J. Taiwan Inst. Chem. Eng. 2014, 45, 233–241. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Willershausen, D.; Ashaghi, K.S.; Engel, L.; Placido, L.; Mund, P.; Bolduan, P.; Czermak, P. Investigations on the use of different ceramic membranes for efficient oil-field produced water treatment. Desalination 2010, 250, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Salahi, A.; Gheshlaghi, A.; Mohammadi, T.; Madaeni, S.S. Experimental performance evaluation of polymeric membranes for treatment of an industrial oily wastewater. Desalination 2010, 262, 235–242. [Google Scholar] [CrossRef]
- Brant, J.A.; Daniel, U.; Kwan, P. Pilot-scale evaluation of chemical cleaning formulations for organic and biologically fouled microfiltration membranes. Water Qual. Technol. Conf. Expo. 2009, 136, 1111–1127. [Google Scholar]
- Bansal, B.; Al-Ali, R.; Prieto, R.M.; Chen, X. Rinsing and cleaning of α-lactalbumin fouled MF membranes. Sep. Purif. Technol. 2006, 48, 202–207. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Song, P.; Wang, X. NaCl cleaning of 0.1 μm polyvinylidene fluoride (PVDF) membrane fouled with humic acid (HA). Chem. Eng. Res. Des. 2018, 132, 325–337. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Lee, K.; Chung, J.-H. Optimum cleaning-in-place conditions for stainless steel microfiltration membrane fouled by terephthalic acid solids. J. Membr. Sci. 2002, 209, 233–240. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Monfared, H.A.; Vatanpour, V.; Shamsabadi, A.A.; Salehi, E.; Daraei, P.; Laki, S.; Khatami, S.M. Coke removal from petrochemical oily wastewater using γ-Al2O3 based ceramic microfiltration membrane. Desalination 2012, 293, 87–93. [Google Scholar] [CrossRef]
- Naim, R.; Levitsky, I.; Gitis, V. Surfactant cleaning of UF membranes fouled by proteins. Sep. Purif. Technol. 2012, 94, 39–43. [Google Scholar] [CrossRef]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical cleaning/disinfection and ageing of organic UF membranes: A review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef]
- Bartlett, M.; Bird, M.; Howell, J. An experimental study for the development of a qualitative membrane cleaning model. J. Membr. Sci. 1995, 105, 147–157. [Google Scholar] [CrossRef]
- Xing, C.-H.; Wen, X.-H.; Qian, Y.; Sun, D.; Klose, P.; Zhang, X. Fouling and cleaning of microfiltration membrane in municipal wastewater reclamation. Water Sci. Technol. 2003, 47, 263–270. [Google Scholar] [CrossRef]



| Membrane Process | Properties |
|---|---|
| Separation | Liquids, particles, molecules, ions, gases, etc. |
| Driving forces | Pressure, concentration, temperature, voltage |
| Configuration | Hollow fibers, flat sheets, tubes, capillary |
| Structure | Charged, solid, porous |
| Morphology | Asymmetric, symmetric |
| Process | MF | UF | NF | RO |
|---|---|---|---|---|
| Selectivity | 0.1–5 µm | 0.01–0.1 µm | 0.001 to 0.01 µm | 0.0001 to 0.001 µm |
| Separation mechanism | Molecular sieve | Solution diffusion | Molecular sieve | Solution diffusion |
| Material retained | Suspended particles, bacteria | Micropollutants, salt, glucose, lactose | Macromolecules, colloids | Dissolved salts |
| Material passed | Water, dissolved solutes | Water, monovalent salts | Water, dissolved salts | Water |
| Materials | Foulant | Cleaning Type | Results | References |
|---|---|---|---|---|
| Mullite ceramic microfiltration membrane (MF) | Crude Oil | Two-step chemical cleaning: Acid (sulfuric acid (H2SO4)), surfactant (sodium dodecyl sulfate (SDS)), chelating agent (ethylene diamine tetraacetic acid (EDTA)), and alkaline (sodium hydroxide (NaOH)) | EDTA and SDS with a concentration of 5 and 10 mM were the best cleaning agents which have flux recovery of about 31.265% and 57.778% Binary solution of SDS + EDTA with the concentration of 5 mM was the best cleaning agent among binary and ternary cleaning solution agents, which led to 41.802% and 65.163% flux recovery | [34] |
| PVDF MF membranes | Organic matter in municipal wastewater | Physical Cleaning: Granules (polyethylene glycol cylindrical granules) and vibration of membrane modules Chemically enhanced backwash (CEB): citric acid (1% (w/v)) was used for CEB. | The single cleaning method did not work. A combination of membrane vibration and agitation of the tank was found to be effective. Physical cleaning efficiently mitigates reversible membrane fouling, and CEB was performed very well for control of irreversible fouling. | [35] |
| PVDF MF membrane | Pomegranate juice | Chemical Cleaning: Various solutions including water (with 0.5, 1, and 1.5% NaOH or 0.1% hydrochloric acid), ethanol (with 77% and 96% purity), and mixture of ethanol (77%) with acetic acid (96%) with 99:01 ratio | The ethanol 77% showed the best performance among different solutions for cleaning | [36] |
| Ceramic MF membrane | Cactus juice | Ultrasonic dental scaler (UDS): operated for 30 min at 29 kHz. Chemical cleaning: s (NaOH and NHO3) at high temperatures (50 and 80 °C), followed by rinsing with pure water | Water flux after cleaning with ultrasound was lower (74.4% at 0.3 bar and 67.74% at 0.5 bar) than the water flux obtained by chemical cleaning | [37] |
| Flat sheet MF membranes with supporting fibers (polyethylene terephthalate (PET)) | Microalgae Nannochloropsis salina | High-Pressure Jet Cleaning: The angle of 70° at a pressure of 130 bar and a cleaning duration of 10 s | This method was restored about 80% of the initial throughput of the membrane. Higher pressure and a longer cleaning duration are supported by a higher throughput of the membrane | [38] |
| Polytetrafluoroethylene (PTFE) MF membrane | Raw wastewater (contained a mass of anionic polyacrylamide (APAM) with the concentration of 749 ± 33 mg L−1, which was used as oil displacement and contained organic matter in large quantity, suspended solids (SS), and salt. | Chemical cleaning: NaOH, NaClO, HCl, HNO3, SDS, and EDTA solutions with a concentration of 0.5% (wt %) were used separately for immersion at 40 °C for 3 h | The cleaning efficiency of 93 percent was achieved by mixing with 0.04 N NaClO + 200 mg L1NaOH, which was found to be better than individual cleaning. Consecutive cleaning with NaClO + NaOH–HCl has also restored 98 percent of the membrane. In addition, the cleaning temperature and time were set at 40C and 3 h. | [39] |
| MF ceramic membrane made from Sayong ball clay | Natural organic matter (NOM) | Chemical Cleaning: NaOH cleaning Membrane sintered at 1050 °C and at a temperature of 25 °C and TMP of 1 bar during filtration | Flux rate improved 1.8 times after chemical cleaning with 0.1M NaOH | [29] |
| MF Membrane | Effluent organic matters (EfOM) (feed waters containing (i) single foulant and (ii) mixed foulants of humic acids, polysaccharides, and protein) | Salt cleaning | The salt cleaning efficiency tends to be more effective for fouling caused by feed water containing more polysaccharide than the other foulants | [40] |
| Fouling Type | Fouling Mechanism |
|---|---|
| Colloidal Fouling | Pore Narrowing, Pore Plugging |
| Organic Fouling | Pore Narrowing, Gel/Cake Formation |
| Inorganic Fouling | Pore narrowing, Gel/Cake Formation |
| Biofouling | Pore Narrowing, Pore Plugging, Gel/Cake Formation–most prominent |
| Process | Foulant | Reference |
|---|---|---|
| Sterile filtration | Cells, Proteins | [42] |
| Beer clarification | Macromolecules of proteins and polysaccharides, minerals, cell debris, protein-polyphenolic aggregates (chill haze), | [27,28] |
| Whey | Protein, fat, ash, lactose, and moisture. | [29,30] |
| Wine clarification | Polysaccharides, polyphenols, and tannic acid | [43] |
| Skim milk filtration | Proteins, minerals, carbohydrates | [44] |
| Oil-in-emulsion filtration | Oil droplets | [45] |
| Cell microfiltration | Protein aggregates | [46] |
| Type of Cleaning Agent | Typical Chemical | Properties | Reference |
|---|---|---|---|
| Caustic | NaOH | Removal of organic (e.g., polysaccharides) and microbial foulants, hydrolysis, solubilization | [66] |
| Alkalis | Carbonates, hydroxides, phosphates | Alteration of surface charges, pH regulation, decrease in the number of bonds between the foulant and the membrane surface | [41,54] |
| Acids | Sulfuric, nitric, citric, and phosphoric (HCl, HNO3, H2SO4, H3PO4, citric, oxalic) | Remove common scaling compounds and metal dioxides, dissolve inorganic precipitates; some acidic hydrolysis of macromolecules (such as: polysaccharides and proteins) | [25,45] |
| Enzymes | Proteases and lipases (a-CT, CP-T, peroxidase) | Hydrolyze e.g., proteins, lipids | [45,52] |
| Surfactants | Anionics, nonionics, cationics (alkyl sulphate, SDS, CTAB) | Dispersion, emulsifying, surface conditioning (modify the surface charge, increase wettability), disrupt functions of bacteria cell walls | [25,41] |
| Sequestrants | Ethylenediamine tetra acetic acid (EDTA), polycarboxylate | Removal of mineral deposits | [67] |
| Disinfectants (and oxidants) | Metabisulphite, NaOCl, peroxyacetic acid, hydrogen peroxide (H2O2), chlorine, and hypochlorite | Increase in hydrophilicity, oxidation of organics, destruction of pathogenic micro-organisms. | [47] |
| Material | Type of Membrane | Type of Foulant | Cleaning Chemical | Results | References |
|---|---|---|---|---|---|
| PET | Hollow fiber MF | Oil from contaminated seawater | Caustic soda, oxalic acid, and sodium hypochlorite | As compared to acid cleaning, alkaline cleaning showed a higher recovery of operating cycle time but a lower recovery of permeate flux. The best-operating cycle time and flux recoveries were achieved using a mixture of alkaline and acid cleaning agents (e.g., 96 percent and 94 percent, respectively). | [68] |
| Ceramic | MF | WPC (whey protein concentrate) powder | Sodium hydroxide (NaOH purity > 99%) (cleaning time (between 5 and 45 min) and transmembrane pressure (from 0.25 to 0.84 bar)) | The bulk of protein fouling was removed within the first few minutes, and the recovery of the flux reached the plateau at a cleaning time of approximately 5 min. | [58] |
| PVDF | MF | Whey | HCL, NaOH, Triton-X100 (cleaning time (30 min.), stirring speeds (400 rpm.) without applying pressure) | Acids showed more efficiency than alkaline to remove mineral compounds. | [68] |
| Ceramic | CF-MF | Commercial rough beer, beer type A | NaOH, HNO3, Ultrasil 11 (low transmembrane pressure Δp = 0.2 bar, a cross-flow velocity v = 2 m/s (corresponding to Reynolds number Re = 1552), and a constant cleaning temperature Tc 22). | Sodium hydroxide was found to be of the highest cleaning power among the three types of chemicals. | [74] |
| Sintered stainless steel | MF | WPC | NaOH (cleaning time (30 min.), temperature (50 °C), TMP (0.5 bar) and a CFV (1.6 ms−1)). | An optimum concentration was found as 0.2 wt in a low percentage of flow recovery. | [65] |
| PVDF | MF | Organic matter (15 mg/L humic acid), with inorganic matter (1 mg/L Fe and 1 mg/L Mn) and a mixture of organic and inorganic matter (humic acid, Fe and Mn) | NaOH and citric acid solution | The cleaning efficiency was different by changing the two chemicals’ cleaning sequence (acid/base and base/acid). Flux recovery was found 20 percent higher in the base/acid sequence. | [75] |
| PVDF | Hollow module MF | The raw water from the first tank of the Guui pilot plant (i.e., Feed 1) shows relatively low turbidity of 12–55 NTU and a moderate DOC concentration of 2.6–3.0 mg/L. The other feed water (i.e., Feed 2) was collected from the second tank of the plant, to which the concentrate from the first tank was introduced. Feed 2 contained highly concentrated turbid matter, i.e., 343–678 NTU, and DOC compounds, i.e., 5.7–7.8 mg/L. The pH was in the range of 7.1 and 7.5 for Feed 1 and 7.7–8.0 for Feed 2. | NaOCl and NaOH, citric acid | The chemical cleaning procedures resulted in 0.93 of the recovery of the water flow for Feed 1 and 0.74 of the recovery of the water flow for Feed 2. | [76] |
| GVWP PVDF | MF | Raw milk | Nitric acid Hydrochloric acid Sulfuric acid Sodium hypochlorite Sodium hydroxide Potassium hydroxide Ammonium hydroxide Ammonia Ethylenediaminetetraacetic acid (EDTA) Sodium dodecyl sulfate (SDS) Triton X-100 Phosphoric acid 2-Propanol | SDS had superior results either alone or in combination with NaOH as a powerful cleaning agent and EDTA as a chelating agent. The cleaning efficiency of hydrochloride and nitric acids was poor. Sodium hypochlorite as a strong base showed a suitable result for chemical cleaning of protein. It was concluded that EDTA could not be used as a chemical cleaner by itself. | [71] |
| PVDF | Durapore® membrane was used (flat sheet PVDF from MilliporeTM with nominal pore size of 0.22 m) | The model solution was prepared with 3.5 g/L of sodium alginate and 2 g/L of BSA for the single cleaning, and 1 g/L of alginate + 1 g/L of BSA for the other experiments. | NaOCl | Cleaning efficiency varied between single and cyclic (i.e., repeated fouling/cleaning cycles): 1% of NaOCl achieved 95% efficiency in a single cleaning, while only 87% cleaning efficiency was seen during cyclic cleaning | [77] |
| Polyolefin with a pore size of 0.4 µm | MF Membrane | Glass industry wastewater | Ultrasound and Chemical cleaning (EDTA, citric acid, NaOH) | Sonication in a caustic solution achieved maximal flux recovery of more than 95%. | [78] |
| Cellulose acetate (CA) | MF Membrane | Microalgal biomass | NaOCl, NaOH, HNO3 and citric acid | 0.75% NaOCl had the best cleaning performance, and approximately 98% flux recovery was achieved. 0.75% NaOH was less effective, resulting in only 68% flux recovery. | [79] |
| Asymmetric multilayer Al2O3 and TiO2 ceramic | MF Membrane | Oil and grease | NaOH solution, Ultrasil P3-14, Ultrasil P3-10 | The efficiency of chemical cleaning of MF and NF membranes was found in the range of 33 to 61% using various lye solutions | [80] |
| PS (0.1 μm) and PS (0.2 μm) | MF Membrane | Oily wastewater from the Tehran refinery | EDTA, SDS | The findings revealed that combinations of SDS and EDTA could effectively clean fouled polymeric membranes. | [81] |
| PP | CMF Hollow Fibre MF | Organic and biological fouling | *Memclean (proprietary ingredients 25% w/w, citric acid 10% w/w, and water balance), *Lavasol II (High pH 10.5–12, cleaner for removing organic and biological foulants, Phosphate free), *Lavasol IV (Neutral pH 4.5–6.5, cleaner for removing organics and proteins), *Lavasol MF (High pH 10.5–12, cleaner for removing organic and biological foulants), *Minncare (Peracetic acid solution oxidizing agent, oxidizes microbial cell proteins and enzyme systems for biofilm removal, decomposes into oxygen, water, and acetic acid) | Caustic soda, a high pH commercial cleaning solution called Memclean C, and hydrogen peroxide were the best cleaning solutions for extracting organic and biological foulants from membrane fibers and restoring membrane performance. | [82] |
| Tubular ceramic | MF | WPC | Sodium hydroxide (NaOH purity 99%, SDS); nitric acid | It has been observed that sodium hydroxide provides flux recovery through desorption and solubilization of proteins, while nitric acid has a detrimental effect on membrane resistance. | [69] |
| PVDF | MF, UF | Skimmed milk | Sodium hydroxide, hydrochloric acid, citric acid | Chemical cleaning, used in this study, damaged the membranes. | [70] |
| Flat-sheet (PVDF) | MF | Milk solution 1 w% | Chemical cleaning (EDTA) and, ultrasound cleaning | Mixed wave ultrasound had a higher cleaning efficiency than other treatments, whether used alone or in combination with EDTA 1 mMole. There was a synergistic effect when ultrasound was used with EDTA as a cleaning factor. | [45] |
| PVDF | MF | α-lactalbumin powder | Chemical cleaning (NaOH) and, Rinsing | The maximum flux recovery achieved by rinsing only about 6% of pure water flux. Flux recovery increased by up to 90% after the caustic solution was added, indicating that almost all of the remaining deposits inside the pores were cleaned as well. | [83] |
| PVDF | MF | Humic acid (HA) | NaCl | High flux recovery of 94.20% was obtained at NaCl concentration of 100 mM with an agitation speed of 600 rpm and temperature of 35 °C. | [84] |
| PE | MF | Oil from contaminated seawater | Caustic soda, oxalic acid, and sodium hypochlorite | Alkaline cleaning recovered more operating cycle time but less permeate flow than acid cleaning. The best working cycle time and flux recovery were achieved using a combination of alkaline and acid cleaning agents (e.g., 96% and 94%, respectively). | [68] |
| Mullite ceramic | MF | Oily wastewater | Acid (sulfuric acid (H2SO4)), surfactant (sodium dodecyl sulphate (SDS)), chelating agent (ethylene diamine tetraacetic acid (EDTA)) and alkaline (sodium hydroxide (NaOH)). | Sulfuric acid was found as the weakest agent to remove foulants. SDS with a concentration of 10 mM utilized 57.78% flux recovery. SDS + EDTA solution with a concentration of 5 mM was the best cleaning agent between the dual and triple cleaning agents, providing a flow recovery of 41.802% and 65.163%, respectively. | [34] |
| Stainless steel tubular membrane, 316 L stainless steel tube surface-coated with a sintered TiO2 layer | MF | Terephthalic acid solids | Sodium hydroxide (NaOH), Ultrasil 10 (Henkel), sodium dodecyl sulfate (SDS), and Tween 80 | Flux recovery increased when the NaOH concentration raised above the range of 3-4 percent (w/v) NaOH but decreased when the NaOH concentration grown above 4 percent. The addition of surfactants (SDS and Tween 80) to the caustic cleaning agent resulted in a significant reduction in cleaning efficiency. | [85] |
| PAN | MF | Activated sludge and yeast suspension | NaOCl, SDS, and NaOH | When compared to SDS and NaOH, the cleaning efficiency of NaOCl was found to be superior. | [72] |
| Ceramic | MF | Coke particles, oily wastewater | 0.1 M HCl, 0.1 NaOH, and 1 wt.% SDS | The best cleaning agent was 0.1 M NaOH solution, which provided the highest flux recovery (80%). As a result, NaOH provided a normal flux recovery, while HCl failed to provide an adequate flux recovery. | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gul, A.; Hruza, J.; Yalcinkaya, F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers 2021, 13, 846. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13060846
Gul A, Hruza J, Yalcinkaya F. Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review. Polymers. 2021; 13(6):846. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13060846
Chicago/Turabian StyleGul, Aysegul, Jakub Hruza, and Fatma Yalcinkaya. 2021. "Fouling and Chemical Cleaning of Microfiltration Membranes: A Mini-Review" Polymers 13, no. 6: 846. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13060846