Antibacterial and Bonding Properties of Universal Adhesive Dental Polymers Doped with Pyrogallol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Antibacterial Activity
2.2.1. Bacterial Strain
2.2.2. Agar Diffusion Test (ADT)
2.2.3. Direct Contact Test (DCT)
2.3. Release Kinetics of PY and the pH Measurements
2.4. Bond Strength Test
2.5. Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis
3. Results
3.1. Antibacterial Activity (ADT and DCT)
3.2. Release Kinetics of the PY and pH Changes
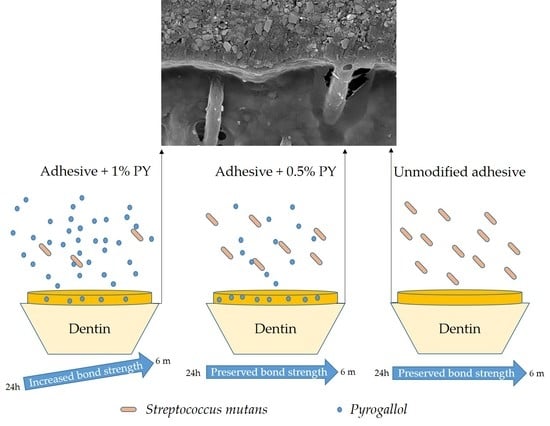
3.3. Shear Bond Strength (SBS)
3.4. SEM Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zarow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suárez, C.-E. Bond Strength of Universal Adhesives to Dentin: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Bourgi, R.; Daood, U.; Bijle, M.N.; Fawzy, A.; Ghaleb, M.; Hardan, L. Reinforced Universal Adhesive by Ribose Crosslinker: A Novel Strategy in Adhesive Dentistry. Polymers 2021, 13, 704. [Google Scholar] [CrossRef]
- Kharouf, N.; Mancino, D.; Rapp, G.; Zghal, J.; Arntz, Y.; Haikel, Y.; Reitzer, F. Does Etching of the Enamel with the Rubbing Technique Promote the Bond Strength of a Universal Adhesive System? J. Contemp. Dent. Pract. 2020, 21, 1117–1121. [Google Scholar] [CrossRef]
- Sauro, S.; Makeeva, I.; Faus-Matoses, V.; Foschi, F.; Giovarruscio, M.; Maciel Pires, P.; Martins Moura, M.E.; Almeida Neves, A.; Faus-Llácer, V. Effects of Ions-Releasing Restorative Materials on the Dentine Bonding Longevity of Modern Universal Adhesives after Load-Cycle and Prolonged Artificial Saliva Aging. Materials 2019, 12, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharouf, N.; Rapp, G.; Mancino, D.; Hemmerlé, J.; Haikel, Y.; Reitzer, F. Effect of etching the coronal dentin with the rubbing technique on the microtensile bond strength of a universal adhesive system. Dent. Med. Probl. 2019, 56, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Frassetto, A.; Breschi, L.; Turco, G.; Marchesi, G.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Cadenaro, M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, e41–e53. [Google Scholar] [CrossRef] [PubMed]
- Abuna, G.; Feitosa, V.P.; Correr, A.B.; Cama, G.; Giannini, M.; Sinhoreti, M.A.; Pashley, D.H.; Sauro, S. Bonding performance of experimental bioactive/biomimetic self-etch adhesives doped with calcium-phosphate fillers and biomimetic analogs of phosphoproteins. J. Dent. 2016, 52, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Suúrez, C.E.; Da Rosa, W.L.O.; Lund, R.G.; Da Silva, A.F.; Piva, E. Bonding Performance of Universal Adhesives: An Updated Systematic Review and Meta-Analysis. J. Adhes. Dent. 2019, 21, 7–26. [Google Scholar] [CrossRef]
- Feitosa, V.P.; Sauro, S.; Zenobi, W.; Silva, J.C.; Abuna, G.; Van Meerbeek, B.; Sinhoreti, M.A.C.; Correr, A.B.; Yoshihara, K. Degradation of Adhesive-Dentin Interfaces Created Using Different Bonding Strategies after Five-year Simulated Pulpal Pressure. J. Adhes. Dent. 2019, 21, 199–207. [Google Scholar]
- Brännström, M.; Nordenvall, K.J. Bacterial penetration, pulpal reaction and the inner surface of Concise enamel bond. Composite fillings in etched and unetched cavities. J. Dent. Res. 1978, 57, 3–10. [Google Scholar] [CrossRef]
- Türkün, L.S.; Ateş, M.; Türkün, M.; Uzer, E. Antibacterial activity of two adhesive systems using various microbiological methods. J. Adhes. Dent. 2005, 7, 315–320. [Google Scholar] [PubMed]
- Kim, J.S.; Shin, D.H. Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin. Restor. Dent. Endod. 2013, 38, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerstein, O.; Matalon, S.; Slutzky, H.; Weiss, E.I. Antibacterial properties of self-etching dental adhesive systems. J. Am. Dent. Assoc. 2007, 138, 349–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Perdigão, J.; Reis, A.; Loguercio, A.D. Dentin adhesion and MMPs: A comprehensive review. J. Esthet. Restor. Dent. 2013, 25, 219–241. [Google Scholar] [CrossRef]
- Suzuki, T.Y.U.; Gallego, J.; Assuncão, W.G.; Briso, A.L.F.; Dos Santos, P.H. Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLoS ONE 2019, 14, e0217750. [Google Scholar] [CrossRef] [Green Version]
- Perdigão, J. Current perspectives on dental adhesion: (1) Dentin adhesion–not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef]
- Takamizawa, T.; Imai, A.; Hirokane, E.; Tsujimoto, A.; Barkmeier, W.W.; Erickson, R.L.; Latta, M.A.; Miyazaki, M. SEM observation of novel characteristic of the dentin bond interfaces of universal adhesives. Dent. Mater. 2019, 35, 1791–1804. [Google Scholar] [CrossRef]
- Bourgi, R.; Hardan, L.; Rivera-Gonzaga, A.; Cuevas-Suárez, C.E. Effect of warm-air stream for solvent evaporation on bond strength of adhesive systems: A systematic review and meta-analysis of in vitro studies. Int. J. Adhes. Adhes. 2021, 105, 102794. [Google Scholar] [CrossRef]
- Hass, V.; Luque-Martinez, I.V.; Gutierrez, M.F. Collagen cross-linkers on dentin bonding: Stability of the adhesive interfaces, degree of conversion of the adhesive, cytotoxicity and in situ MMP inhibition. Dent. Mater. 2016, 32, 732–741. [Google Scholar] [CrossRef]
- Kharouf, N.; Haikel, Y.; Ball, V. Polyphenols in Dental Applications. Bioengineering 2020, 7, 72. [Google Scholar] [CrossRef]
- Kim, S.R.; Shin, D.H. Antibacterial effect of self-etching adhesive systems on Streptococcus mutans. Restor. Dent. Endod. 2014, 39, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Slutzky, H.; Matalon, S.; Weiss, E.I. Antibacterial surface properties of polymerized single-bottle bonding agents: Part II. Quintessence Int. 2004, 35, 275–279. [Google Scholar] [PubMed]
- ITENA Clinical Products. Available online: https://www.itena-clinical.com/fr/adhesion/65-iperbond-max.html (accessed on 11 March 2021).
- Kharouf, N.; Zghal, J.; Addiego, F.; Gabelout, M.; Jmal, H.; Haïkel, J.; Bahlouli, N.; Ball, V. Tannic acid speeds up the setting of mineral trioxide aggregate cements and improves its surface and bulk properties. J. Colloid. Interface. Sci. 2021, 589, 318–326. [Google Scholar] [CrossRef]
- Shin, M.; Park, E.; Lee, H. Plant-inspired pyrogallol-containing functional materials. Adv. Funct. Mater. 2019, 29, 1903022. [Google Scholar] [CrossRef]
- McManus, J.P.; Davis, K.G.; Beart, J.E.; Gaffney, S.H.; Lilley, T.H.; Haslam, E. Polyphenol interactions. Part 1. Introduction; some observations on the reversible complexation of polyphenols with proteins and polysaccharides. J. Chem. Soc. Perkin Trans. 2 1985, 28, 1429–1438. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Barcellos, D.C.; Silva, T.M.D.; Borges, A.L.S.; Cavalcanti, B.D.N.; Prakki, A.; Oliveira, H.P.M.; Gonçalves, S.E.P. Mechanical-physicochemical properties and biocompatibility of catechin-incorporated adhesive resins. J. Appl. Oral. Sci. 2019, 27, e20180111. [Google Scholar] [CrossRef]
- Daood, U.; Swee Heng, C.; Neo Chiew Lian, J.; Fawzy, A.S. In vitro analysis of riboflavin-modified, experimental, two-step etch-and-rinse dentin adhesive: Fourier transform infrared spectroscopy and micro-Raman studies. Int. J. Oral. Sci. 2015, 7, 110–124. [Google Scholar] [CrossRef]
- Neri, J.R.; Yamauti, M.; Feitosa, V.P.; Pires, A.P.; Araújo Rdos, S.; Santiago, S.L. Physicochemical properties of a methacrylate-based dental adhesive incorporated with epigallocatechin-3-gallate. Braz. Dent. J. 2014, 25, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharouf, N.; Arntz, Y.; Eid, A.; Zghal, J.; Sauro, S.; Haikel, Y.; Mancino, D. Physicochemical and Antibacterial Properties of Novel, Premixed Calcium Silicate-Based Sealer Compared to Powder–Liquid Bioceramic Sealer. J. Clin. Med. 2020, 9, 3096. [Google Scholar] [CrossRef]
- Kharouf, N.; Ashi, T.; Eid, A.; Maguina, L.; Zghal, J.; Sekayan, N.; Bourgi, R.; Hardan, L.; Sauro, S.; Haikel, Y.; et al. Does Adhesive Layer Thickness and Tag Length Influence Short/Long-Term Bond Strength of Universal Adhesive Systems? An In-Vitro Study. Appl. Sci. 2021, 11, 2635. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Costa, P.; Sousa, J.M. Modelling and comparison of dissolution profiles. Eur. J. Pharm. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Mickenautsch, S.; Mount, G.; Yengopal, V. Therapeutic effect of glass-ionomers: An overview of evidence. Aust. Dent. J. 2011, 56, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lapinska, B.; Konieczka, M.; Zarzycka, B.; Sokolowski, K.; Grzegorczyk, J.; Lukomska-Szymanska, M. Flow Cytometry Analysis of Antibacterial Effects of Universal Dentin Bonding Agents on Streptococcus mutans. Molecules 2019, 24, 532. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Huang, X.; Huang, C.; Wang, Y.; Zhang, Y. Epigallocatechin-3-gallate (EGCG) enhances the therapeutic activity of a dental adhesive. J. Dent. 2012, 40, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Mankovskaia, A.; Lévesque, C.M.; Prakki, A. Catechin-incorporated dental copolymers inhibit growth of Streptococcus mutans. J. Appl. Oral Sci. 2013, 21, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Dávila-Sánchez, A.; Gutierrez, M.F.; Bermudez, J.P.; Méndez-Bauer, L.; Pulido, C.; Kiratzc, F.; Alegria-Acevedo, L.F.; Farago, P.V.; Loguercio, A.D.; Sauro, S.; et al. Effects of Dentine Pretreatment Solutions Containing Flavonoids on the Resin Polymer-Dentine Interface Created Using a Modern Universal Adhesive. Polymers 2021, 13, 1145. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.M.; Manso, A.P.; Geraldeli, S.; Tay, F.R.; Pashley, D.H. Durability of bonds and clinical success of adhesive restorations. Dent. Mater. 2012, 28, 72–86. [Google Scholar] [CrossRef] [Green Version]
- Tjaderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef]
- Hannas, A.R.; Pereira, J.C.; Granjeiro, J.M.; Tjaderhane, L. The role of matrix metalloproteinases in the oral environment. Acta Odontol. Scand. 2007, 65, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tjaderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef] [Green Version]
- Gomes, G.M.; Gomes, O.M.; Reis, A.; Gomes, J.C.; Loguercio, A.D.; Calixto, A.L. Effect of operator experience on the outcome of fiber post cementation with different resin cements. Oper. Dent. 2013, 38, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Sano, H.; Chowdhury, A.F.-M.A.; Saikaew, P.; Matsumoto, M.; Hoshika, S.; Yamauti, M. The microtensile bond strength test: Its historical background and application to bond testing. Jpn. Dent. Sci. Rev. 2020, 56, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Beloica, M.; Goracci, C.; Carvalho, C.-A.; Radovic, I.; Margvelashvili, M.; Vulicevic, Z.-R.; Ferrari, M. Microtensile vs. microshear bond strength of all-in-one adhesives to unground enamel. J. Adhes. Dent. 2010, 12, 427–433. [Google Scholar]
- Gotti, V.B.; Feitosa, V.P.; Sauro, S.; Correr-Sobrinho, L.; Leal, F.B.; Stansbury, J.W.; Correr, A.B. Effect of antioxidants on the dentin interface bond stability of adhesives exposed to hydrolytic degradation. J. Adhes. Dent. 2015, 17, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Shamsedin, M.; Arash, V.; Jahromi, M.B.; Moghadamnia, A.A.; Kamel, M.R.; Ezoji, F.; Bijani, A.; Kavoli, S.; Ghasemi, T.; Ramezani, G. Efficacy of quercetin flavonoid in recovering the postbleaching bond strength of orthodontic brackets: A preliminary study. J. Orthod. Sci. 2017, 6, 16–21. [Google Scholar] [PubMed]
- Yang, H.; Li, K.; Yan, H.; Liu, S.; Wang, Y.; Huang, C. High-performance therapeutic quercetin-doped adhesive for adhesive-dentin interfaces. Sci. Rep. 2017, 7, 8189. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Material | Composition | Applications |
|---|---|---|
| Iperbond Max (Itena Clinical, Paris, France) | 10-MDP, 4-META, methacrylates, photo-initiators, ethanol, water, fumed silica | Apply (20 s) Wait until the solvent had completely evaporated (20 s) Dry (5 s) Light cure (10 s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharouf, N.; Eid, A.; Hardan, L.; Bourgi, R.; Arntz, Y.; Jmal, H.; Foschi, F.; Sauro, S.; Ball, V.; Haikel, Y.; et al. Antibacterial and Bonding Properties of Universal Adhesive Dental Polymers Doped with Pyrogallol. Polymers 2021, 13, 1538. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101538
Kharouf N, Eid A, Hardan L, Bourgi R, Arntz Y, Jmal H, Foschi F, Sauro S, Ball V, Haikel Y, et al. Antibacterial and Bonding Properties of Universal Adhesive Dental Polymers Doped with Pyrogallol. Polymers. 2021; 13(10):1538. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101538
Chicago/Turabian StyleKharouf, Naji, Ammar Eid, Louis Hardan, Rim Bourgi, Youri Arntz, Hamdi Jmal, Federico Foschi, Salvatore Sauro, Vincent Ball, Youssef Haikel, and et al. 2021. "Antibacterial and Bonding Properties of Universal Adhesive Dental Polymers Doped with Pyrogallol" Polymers 13, no. 10: 1538. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13101538