Unfolding of Helical Poly(L-Glutamic Acid) in N,N-Dimethylformamide Probed by Pyrene Excimer Fluorescence (PEF)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Steady-State Fluorescence
2.3. Time-Resolved Fluorescence
2.4. Fluorescence Decay Analysis
3. Results
3.1. Steady-State Fluorescence
3.2. Time-Resolved Fluorescence
3.3. Fluorescence Blob Model Analysis of Decays
4. Discussion
4.1. Unfolding of a Protein According to the Two-State Model
4.2. Using <Nblob> as a Structural Parameter
4.3. Strengths and Weaknesses of PEF-Based Macromolecular Structure Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathew, A.; Siu, H.; Duhamel, J. A Blob Model to Study Chain Folding by Fluorescence. Macromolecules 1999, 32, 7100–7108. [Google Scholar] [CrossRef]
- Duhamel, J. Polymer Chain Dynamics in Solution Probed with a Fluorescence Blob Model. Acc. Chem. Res. 2006, 39, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, J. New Insights in the Study of Pyrene Excimer Fluorescence to Characterize Macromolecules and their Supramolecular Assemblies in Solution. Langmuir 2012, 28, 6527–6538. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, J. Global Analysis of Fluorescence Decays to Probe the Internal Dynamics of Fluorescently Labeled Macromolecules. Langmuir 2014, 30, 2307–2324. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, S.; Duhamel, J. Long Range Polymer Chain Dynamics Studied by Fluorescence Quenching. Macromolecules 2016, 49, 6149–6162. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.; Whitton, G.; Casier, R.; Gauthier, M.; Duhamel, J. Arborescent Poly(L-glutamic acid)s as Standards to Study the Dense Interior of Polypeptide Mesoglobules by Pyrene Excimer Fluorescence. Macromolecules 2018, 51, 7914–7923. [Google Scholar] [CrossRef]
- Li, L.; Duhamel, J. Conformation of Pyrene-Labeled Amylose in DMSO Characterized with the Fluorescence Blob Model. Macromolecules 2016, 49, 7965–7974. [Google Scholar] [CrossRef] [Green Version]
- Duhamel, J.; Kanagalingam, S.; O’Brien, T.; Ingratta, M. Side-Chain Dynamics of an α–Helical Polypeptide Monitored by Fluorescence. J. Am. Chem. Soc. 2003, 125, 12810–12822. [Google Scholar] [CrossRef]
- Ingratta, M.; Duhamel, J. Effect of Side-chain Length on the Side-chain Dynamics of α–Helical Poly(L-glutamic acid) as Probed by a Fluorescence Blob Model. J. Phys. Chem. B 2008, 112, 9209–9218. [Google Scholar] [CrossRef] [PubMed]
- Casier, R.; Duhamel, J. Pyrene Excimer Fluorescence as a Direct and Easy Experimental Means to Characterize the Length Scale and Dynamics of Polypeptide Foldons. Macromolecules 2018, 51, 3450–3457. [Google Scholar] [CrossRef]
- Casier, R.; Duhamel, J. The Effect of Structure on Polypeptide Blobs: A Model Study Using Poly(L-Lysine). Langmuir 2020, 36, 7980–7990. [Google Scholar] [CrossRef] [PubMed]
- Casier, R.; Duhamel, J. The Effect of Like-Charges on the Conformation and Internal Dynamics of Polypeptides Probed by Pyrene Excimer Fluorescence. Macromolecules 2020, 53, 5147–5157. [Google Scholar] [CrossRef]
- Li, L.; Kim, D.; Zhai, X.; Duhamel, J. A Pyrene Excimer Fluorescence (PEF) Study of the Interior of Amylopectin in Dilute Solution. Macromolecules 2020, 53, 6850–6860. [Google Scholar] [CrossRef]
- Li, L.; Duhamel, J. Interior of Amylopectin and Nanosized Amylopectin Fragments Probed by Viscometry, Dynamic Light Scattering, and Pyrene Excimer Formation. Polymers 2020, 12, 2649. [Google Scholar] [CrossRef] [PubMed]
- Casier, R.; Duhamel, J. Blob-Based Approach to Estimate the Folding Time of Proteins Supported by Pyrene Excimer Fluorescence Experiments. Macromolecules 2020, 53, 9823–9835. [Google Scholar] [CrossRef]
- Casier, R.; Duhamel, J. Blob-Based Predictions of Protein Folding Times from the Amino Acid Dependent Conformation of Polypeptides in Solution. Macromolecules 2021, 54, 919–929. [Google Scholar] [CrossRef]
- Schatz, C.; Pichot, C.; Delair, T.; Viton, C.; Domard, A. Static Light Scattering Studies on Chitosan Solutions: From Macromolecular Chains to Colloidal Dispersions. Langmuir 2003, 19, 9896–9903. [Google Scholar] [CrossRef]
- Rajapaksha, A.; Stanley, C.B.; Todd, B.A. Effects of Macromolecular Crowding on the Structure of a Protein Complex: A Small Angle Scattering Study of Peroxide Dismutase. Biophys. J. 2015, 108, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, M.; Nakano, R.; Jinbo, Y.; Saito, Y.; Ohno, S.; Togashi, D.; Enomoto, K.; Narumi, A.; Haba, O.; Kawaguchi, S. Graft Density Dependence of Main Chain Stiffness in Molecular Rod Brushes. Macromolecules 2015, 48, 5878–5886. [Google Scholar] [CrossRef]
- Greene, R.F.; Pace, C.N. Urea and Guanidinium Hydrochloride Denaturation of Ribonuclease, Lysozyme, a-Chimotrypsin, and b-Lactoglobulin. J. Biol. Chem. 1974, 249, 5388–5393. [Google Scholar] [CrossRef]
- Santoro, M.M.; Bolen, D.W. Unfolding Free Energy Changes Determined by the Linear Extrapolation Method. 1. Unfolding of Phenylmethanesulfonyl a-Chymotrypsin Using Different Denaturants. Biochemistry 1988, 27, 8063–8068. [Google Scholar] [CrossRef]
- Santoro, M.M.; Bolen, D.W. A Test of the Linear Extrapolation of Unfolding Free Energy Changes over an Extended Denaturant Concentration Range. Biochemistry 1992, 31, 4901–4907. [Google Scholar] [CrossRef]
- Smith, J.S.; Scholtz, J.M. Guanidine Hydrochloride Unfolding of Peptide Helices: Separation of Denaturant and Salt Effects. Biochemistry 1996, 35, 7292–7297. [Google Scholar] [CrossRef]
- Curnow, P.; Booth, P.J. Combined Kinetic and Thermodynamic Analysis of a-Helical Membrane Protein Unfolding. Proc. Natl. Acad. Sci. USA 2007, 104, 18970–18975. [Google Scholar] [CrossRef] [Green Version]
- Findlay, H.E.; Rutherford, N.G.; Henderson, P.J.F.; Booth, P.J. Unfolding Free Energy of a Two Domain Transmembrane Sugar Transport Protein. Proc. Natl. Acad. Sci. USA 2010, 107, 18451–18456. [Google Scholar] [CrossRef] [Green Version]
- Patra, M.; Mukhopadhyay, C.; Chakrabarti, A. Probing Conformational Stability and Dynamics of Erythroid and Nonerythroid Spectrin: Effect of Urea and Guanidine Hydrochloride. PLoS ONE 2015, 10, e0116991. [Google Scholar] [CrossRef]
- Tanford, C.; Kawahara, K.; Lapanje, S. Proteins as Random Coils. I. Intrinsic Viscosity and Sedimentation Coefficients in Concentrated Guanidine Hydrochloride. J. Am. Chem. Soc. 1967, 89, 729–736. [Google Scholar] [CrossRef]
- Flecha, F.L.G. Kinetic Stability of Membrane Proteins. Biophys. Rev. 2017, 9, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Kazlauskas, R. Engineering more Stable Proteins. Chem. Soc. Rev. 2018, 47, 9026–9045. [Google Scholar] [CrossRef]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A.; Vetterling, W.T. Numerical Recipes. The Art of Scientific Computing (Fortran Version); Cambridge University Press: Cambridge, UK, 1992; p. 82. [Google Scholar]
- Yamaoka, K.; Ueda, K. Reversing-Pulse Electric Birefringence Study of Helical Poly(a-L-glutamic acid) in N,N-Dimethylformamide with Emphasis on a New Data Analysis for the Polydisperse System. J. Phys. Chem. 1982, 86, 406–413. [Google Scholar] [CrossRef]
- Cuniberti, C.; Perico, A. Intramolecular Excimer Formation in Polymers. Pyrene-Labeled Polyvinylacetate. Eur. Polym. J. 1980, 16, 887–893. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Acad.: New York, NY, USA, 1999; p. 241. [Google Scholar]
- Bedouelle, P. Principles and Equations for Measuring and Interpreting Protein Stability: From Monomer to Tetramer. Biochimie 2016, 121, 29–37. [Google Scholar] [CrossRef]
- Karshikoff, A.; Ladenstein, R. Ion Pairs and the Thermotolerance of Proteins from Hyperthermophiles: A ‘Traffic Rule’ for Hot Roads. Trends Biochem. Sci. 2001, 26, 550–557. [Google Scholar] [CrossRef]
- Kumar, S.; Nussinov, R. Close-Range Electrostatic Interactions in Proteins. ChemBioChem 2002, 3, 604–617. [Google Scholar] [CrossRef]
- Fass, D. Disulfide Bonding in Protein Biophysics. Annu. Rev. Biophys. 2012, 41, 63–79. [Google Scholar] [CrossRef]
- Pace, N.C.; Scholtz, J.M.; Grimsley, G.R. Forces Stabilizing Proteins. FEBS Lett. 2014, 588, 2177–2184. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L.; Corey, R.B.; Branson, H.R. The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain. Proc. Natl. Acad. Sci.USA 1951, 37, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Winnik, F.M.; Winnik, F.M. Photophysics of Preassociated Pyrenes in Aqueous Polymer Solutions and in Other Organized Media. Chem. Rev. 1993, 93, 587–614. [Google Scholar] [CrossRef]

 ) 4.4, (
) 4.4, (  ) 4.9, (
) 4.9, (  ) 6.9, (
) 6.9, (  ) 9.0, and (
) 9.0, and (  ) 14.3 mol%, and (D) Py(x)-PDLGA, where x = (
) 14.3 mol%, and (D) Py(x)-PDLGA, where x = (  ) 6.0, (
) 6.0, (  ) 8.0, (
) 8.0, (  ) 10.4, (
) 10.4, (  ) 11.3, and (
) 11.3, and (  ) 12.3 mol%.
) 12.3 mol%.
 ) 4.4, (
) 4.4, (  ) 4.9, (
) 4.9, (  ) 6.9, (
) 6.9, (  ) 9.0, and (
) 9.0, and (  ) 14.3 mol%, and (D) Py(x)-PDLGA, where x = (
) 14.3 mol%, and (D) Py(x)-PDLGA, where x = (  ) 6.0, (
) 6.0, (  ) 8.0, (
) 8.0, (  ) 10.4, (
) 10.4, (  ) 11.3, and (
) 11.3, and (  ) 12.3 mol%.
) 12.3 mol%.

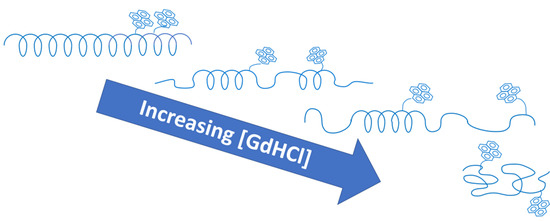
 ) 0 M, (
) 0 M, (  ) 0.3 M, (
) 0.3 M, (  ) 0.7 M, (
) 0.7 M, (  ) 1 M, (
) 1 M, (  ) 3 M, and (
) 3 M, and (  ) 5M GdHCl. (C) Plot of <Nblob> as a function of GdHCl concentration for (
) 5M GdHCl. (C) Plot of <Nblob> as a function of GdHCl concentration for (  ) PLGA and (
) PLGA and (  ) PDLGA.
) PDLGA.
 ) 0 M, (
) 0 M, (  ) 0.3 M, (
) 0.3 M, (  ) 0.7 M, (
) 0.7 M, (  ) 1 M, (
) 1 M, (  ) 3 M, and (
) 3 M, and (  ) 5M GdHCl. (C) Plot of <Nblob> as a function of GdHCl concentration for (
) 5M GdHCl. (C) Plot of <Nblob> as a function of GdHCl concentration for (  ) PLGA and (
) PLGA and (  ) PDLGA.
) PDLGA.
 ) 0 M, (
) 0 M, (  ) 0.3 M, (
) 0.3 M, (  ) 0.7 M, (
) 0.7 M, (  ) 1 M, (
) 1 M, (  ) 3 M, and (
) 3 M, and (  ) 5 M GdHCl. (C) Plot of kblob as a function of GdHCl concentration for (
) 5 M GdHCl. (C) Plot of kblob as a function of GdHCl concentration for (  ) PLGA and (
) PLGA and (  ) PDLGA.
) PDLGA.
 ) 0 M, (
) 0 M, (  ) 0.3 M, (
) 0.3 M, (  ) 0.7 M, (
) 0.7 M, (  ) 1 M, (
) 1 M, (  ) 3 M, and (
) 3 M, and (  ) 5 M GdHCl. (C) Plot of kblob as a function of GdHCl concentration for (
) 5 M GdHCl. (C) Plot of kblob as a function of GdHCl concentration for (  ) PLGA and (
) PLGA and (  ) PDLGA.
) PDLGA.

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Casier, R.; Duhamel, J. Unfolding of Helical Poly(L-Glutamic Acid) in N,N-Dimethylformamide Probed by Pyrene Excimer Fluorescence (PEF). Polymers 2021, 13, 1690. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111690
Yuan W, Casier R, Duhamel J. Unfolding of Helical Poly(L-Glutamic Acid) in N,N-Dimethylformamide Probed by Pyrene Excimer Fluorescence (PEF). Polymers. 2021; 13(11):1690. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111690
Chicago/Turabian StyleYuan, Weize, Remi Casier, and Jean Duhamel. 2021. "Unfolding of Helical Poly(L-Glutamic Acid) in N,N-Dimethylformamide Probed by Pyrene Excimer Fluorescence (PEF)" Polymers 13, no. 11: 1690. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111690