Novel Self-Healing Metallocopolymers with Pendent 4-Phenyl-2,2′:6′,2″-terpyridine Ligand: Kinetic Studies and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Analytical Methods
2.2.1. Characterization of the Structure and Composition of the Polymers
2.2.2. Study of Kinetics of Copolymerization
2.2.3. Mechanical Properties
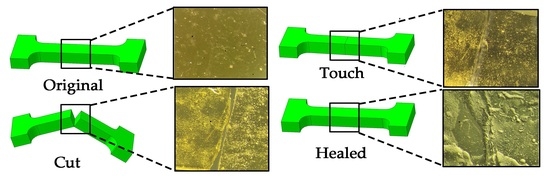
2.2.4. Self-Healing Properties
2.3. Synthesis of Copolymers
3. Results and Discussion
3.1. Characterization of the Structure and Composition of the Polymers
3.2. Study of Kinetics of Copolymerization
3.3. Study of Self-Healing and Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bode, S.; Bose, R.K.; Matthes, S.; Ehrhardt, M.; Seifert, A.; Schacher, F.H.; Paulus, R.M.; Stumpf, S.; Sandmann, B.; Vitz, J.; et al. Self-Healing Metallopolymers Based on Cadmium Bis(Terpyridine) Complex Containing Polymer Networks. Polym. Chem. 2013, 4, 4966–4973. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Yadav, B.C.; Singh, S.; Uflyand, I.E. Self-Healing and Shape Memory Metallopolymers: State-of-the-Art and Future Perspectives. Dalton Trans. 2020, 49, 3042–3087. [Google Scholar] [CrossRef]
- Ahner, J.; Bode, S.; Micheel, M.; Dietzek, B.; Hager, M.D. Self-Healing Functional Polymeric Materials. In Self-Healing Materials; Hager, M.D., van der Zwaag, S., Schubert, U.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 247–283. ISBN 978-3-319-32778-5. [Google Scholar]
- Bergman, S.D.; Wudl, F. Mendable Polymers. J. Mater. Chem. 2008, 18, 41–62. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-Healing Polymeric Materials Based on Microencapsulated Healing Agents: From Design to Preparation. Prog. Polym. Sci. 2015, 49–50, 175–220. [Google Scholar] [CrossRef]
- Du, G.; Mao, A.; Yu, J.; Hou, J.; Zhao, N.; Han, J.; Zhao, Q.; Gao, W.; Xie, T.; Bai, H. Nacre-Mimetic Composite with Intrinsic Self-Healing and Shape-Programming Capability. Nat. Commun. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabani, P.; Shokrieh, M.M.; Zibaei, I. Effect of the Conversion Degree and Multiple Healing on the Healing Efficiency of a Thermally Reversible Self-Healing Polymer. Polym. Adv. Technol. 2019, 30, 2906–2917. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, X.; Yang, P.; Li, J.; Zhang, D.; Liu, L.; Wang, Y. The Influence of Furanyl Monomer on the Self-Healing Polyurethanes by Reverse Diels-Alder Cross-Link. Polym. Adv. Technol. 2019, 30, 804–807. [Google Scholar] [CrossRef]
- Jian, X.; Hu, Y.; Zhou, W.; Xiao, L. Self-Healing Polyurethane Based on Disulfide Bond and Hydrogen Bond. Polym. Adv. Technol. 2018, 29, 463–469. [Google Scholar] [CrossRef]
- Chung, C.-M.; Roh, Y.-S.; Cho, S.-Y.; Kim, J.-G. Crack Healing in Polymeric Materials via Photochemical [2+2] Cycloaddition. Chem. Mater. 2004, 16, 3982–3984. [Google Scholar] [CrossRef]
- Hart, L.R.; Nguyen, N.A.; Harries, J.L.; Mackay, M.E.; Colquhoun, H.M.; Hayes, W. Perylene as an Electron-Rich Moiety in Healable, Complementary π–π Stacked, Supramolecular Polymer Systems. Polymer 2015, 69, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Kuhl, N.; Bode, S.; Bose, R.K.; Vitz, J.; Seifert, A.; Hoeppener, S.; Garcia, S.J.; Spange, S.; van der Zwaag, S.; Hager, M.D.; et al. Acylhydrazones as Reversible Covalent Crosslinkers for Self-Healing Polymers. Adv. Funct. Mater. 2015, 25, 3295–3301. [Google Scholar] [CrossRef]
- Zhang, D.-L.; Ju, X.; Li, L.-H.; Kang, Y.; Gong, X.-L.; Li, B.-J.; Zhang, S. An Efficient Multiple Healing Conductive Composite via Host–Guest Inclusion. Chem. Commun. 2015, 51, 6377–6380. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.K.; Hohlbein, N.; Garcia, S.J.; Schmidt, A.M.; van der Zwaag, S. Connecting Supramolecular Bond Lifetime and Network Mobility for Scratch Healing in Poly(Butyl Acrylate) Ionomers Containing Sodium, Zinc and Cobalt. Phys. Chem. Chem. Phys. 2015, 17, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Kersey, F.R.; Loveless, D.M.; Craig, S.L. A Hybrid Polymer Gel with Controlled Rates of Cross-Link Rupture and Self-Repair. J. R. Soc. Interface 2007, 4, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Mansfeld, U.; Winter, A.; Hager, M.D.; Hoogenboom, R.; Günther, W.; Schubert, U.S. Orthogonal Self-Assembly of Stimuli-Responsive Supramolecular Polymers Using One-Step Prepared Heterotelechelic Building Blocks. Polym. Chem. 2013, 4, 113–123. [Google Scholar] [CrossRef]
- Li, C.-H.; Wang, C.; Keplinger, C.; Zuo, J.-L.; Jin, L.; Sun, Y.; Zheng, P.; Cao, Y.; Lissel, F.; Linder, C.; et al. A Highly Stretchable Autonomous Self-Healing Elastomer. Nat. Chem. 2016, 8, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xie, C.; Yu, C.; Fei, G.; Wang, Z.; Xia, H. A Facile Strategy for Self-Healing Polyurethanes Containing Multiple Metal-Ligand Bonds. Macromol Rapid Commun. 2018, 39, e1700678. [Google Scholar] [CrossRef]
- An, R.; Zhang, X.; Han, L.; Wang, X.; Zhang, Y.; Shi, L.; Ran, R. Healing, Flexible, High Thermal Sensitive Dual-Network Ionic Conductive Hydrogels for 3D Linear Temperature Sensor. Mater. Sci. Eng. C 2020, 107, 110310. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, L.; Liao, L. Light and Ferric Ion Responsive Fluorochromic Hydrogels with High Strength and Self-Healing Ability. Polym. Chem. 2019, 10, 6481–6488. [Google Scholar] [CrossRef]
- Kalista, S.J.; Pflug, J.R.; Varley, R.J. Effect of Ionic Content on Ballistic Self-Healing in EMAA Copolymers and Ionomers. Polym. Chem. 2013, 4, 4910–4926. [Google Scholar] [CrossRef]
- Li, T.; Hu, X.; Zhang, Q.; Zhao, Y.; Wang, P.; Wang, X.; Qin, B.; Lu, W. Poly(Acrylic Acid)-Chitosan @ Tannic Acid Double-Network Self-Healing Hydrogel Based on Ionic Coordination. Polym. Adv. Technol. 2020, 31, 1648–1660. [Google Scholar] [CrossRef]
- Jing, Z.; Xu, A.; Liang, Y.-Q.; Zhang, Z.; Yu, C.; Hong, P.; Li, Y. Biodegradable Poly(Acrylic Acid-Co-Acrylamide)/Poly(Vinyl Alcohol) Double Network Hydrogels with Tunable Mechanics and High Self-Healing Performance. Polymers 2019, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.A.; Boydston, A.J.; Bielawski, C.W. Towards Electrically Conductive, Self-Healing Materials. J. R. Soc. Interface 2007, 4, 359–362. [Google Scholar] [CrossRef]
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-Inspired Materials: Self-Healing through Coordination Chemistry. Chem. Eur. J. 2016, 22, 844–857. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, Y.; Xie, Q.; Fan, C.; Hilborn, J.; Dai, J.; Ossipov, D.A. Moldable Hyaluronan Hydrogel Enabled by Dynamic Metal–Bisphosphonate Coordination Chemistry for Wound Healing. Adv. Health Mater. 2018, 7, 1700973. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Hussain, I.; Oderinde, O.; Yao, F.; Zhang, J.; Fu, G. A Conductive Self-Healing Double Network Hydrogel with Toughness and Force Sensitivity. Chem. Eur. J. 2018, 24, 6632–6638. [Google Scholar] [CrossRef]
- Pignanelli, J.; Billet, B.; Straeten, M.; Prado, M.; Schlingman, K.; Ahamed, M.J.; Rondeau-Gagné, S. Imine and Metal–Ligand Dynamic Bonds in Soft Polymers for Autonomous Self-Healing Capacitive-Based Pressure Sensors. Soft Matter 2019, 15, 7654–7662. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Jing, H.; Guo, B.; Zhang, L.; Liu, F. Bioinspired Engineering of Sacrificial Metal–Ligand Bonds into Elastomers with Supramechanical Performance and Adaptive Recovery. Macromolecules 2016, 49, 1781–1789. [Google Scholar] [CrossRef]
- Yang, H.; Wang, A.; Zhang, L.; Zhou, X.; Yang, G.; Li, Y.; Zhang, Y.; Zhang, B.; Song, J.; Feng, Y. Healable Terpyridine-Based Supramolecular Gels and the Luminescent Properties of the Rare Earth Metal Complex. New J. Chem. 2017, 41, 15173–15179. [Google Scholar] [CrossRef]
- Arnedo-Sánchez, L.; Bhowmik, S.; Hietala, S.; Puttreddy, R.; Lahtinen, M.; De Cola, L.; Rissanen, K. Rapid Self-Healing and Anion Selectivity in Metallosupramolecular Gels Assisted by Fluorine–Fluorine Interactions. Dalton Trans. 2017, 46, 7309–7316. [Google Scholar] [CrossRef] [Green Version]
- Kupfer, S.; Zedler, L.; Guthmuller, J.; Bode, S.; Hager, M.D.; Schubert, U.S.; Popp, J.; Gräfe, S.; Dietzek, B. Self-Healing Mechanism of Metallopolymers Investigated by QM/MM Simulations and Raman Spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12422–12432. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Moon, H.H.; Paeng, K.; Song, C. Reversible Assembly of Terpyridine Incorporated Norbornene-Based Polymer via a Ring-Opening Metathesis Polymerization and Its Self-Healing Property. Polymers 2018, 10, 1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pomogailo, A.D. Catalysis by Polymer-Immobilized Metal Complexes; Gordon and Breach Science Publishers: Amsterdam, The Netherlands, 1998; ISBN 978-90-5699-130-2. [Google Scholar]
- Pomogailo, A.D.; Dzhardimalieva, G.I. Problems of Unit Variability in Metal-Containing Polymers. Russ. Chem. Bull. 1998, 47, 2319–2337. [Google Scholar] [CrossRef]
- Pomogailo, A.D. (Co)Polymerization of Metal-Containing Monomers as a Way of MMC Synthesis. Macromol. Symp. 1998, 131, 115–125. [Google Scholar] [CrossRef]
- Tzanetos, N.P.; Andreopoulou, A.K.; Kallitsis, J.K. Side-Chain Terpyridine Polymers through Atom Transfer Radical Polymerization and Their Ruthenium Complexes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4838–4848. [Google Scholar] [CrossRef]
- Moad, G. RAFT Polymerization to Form Stimuli-Responsive Polymers. Polym. Chem. 2017, 8, 177–219. [Google Scholar] [CrossRef]
- Ding, H.; Xiaoxu, L.; Zhang, X.N.; Wu, Z.L.; Li, Z.; Sun, G. Tough Supramolecular Hydrogels with Excellent Self-Recovery Behavior Mediated by Metal-Coordination Interaction. Polymer 2019, 171, 201–210. [Google Scholar] [CrossRef]
- Baimuratova, R.K.; Dzhardimalieva, G.I.; Zhinzhilo, V.A.; Kydralieva, K.A.; Uflyand, I.E. Metal-Containing Monomers Based on Copper and Zinc Salts of Unsaturated Acids and Pendent 4-phenyl-2,2′:6′,2″-Terpyridine Ligands: Synthesis, Characterization and Thermal Properties. Key Eng. Mater. 2020, 869, 119–128. [Google Scholar] [CrossRef]
- Korolev, G.V.; Perepelitsina, E.O. Effect of intermolecular interactions on the kinetics of radical polymerization. Polym. Sci. Ser. B. 1997, 39, 338–341. [Google Scholar]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.-C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid Self-Healing Hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Ge, X.; Qian, Y.; Wei, M.; Zhang, Z.; Yuan, W.-E.; Ouyang, Y. Advances in Synthesis and Applications of Self-Healing Hydrogels. Front Bioeng. Biotechnol. 2020, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, M.; Ren, J.; Wang, J.; Fan, L.; Xu, Q. Preparation and Swelling Properties of Graphene Oxide/Poly(Acrylic Acid-Co-Acrylamide) Super-Absorbent Hydrogel Nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2012, 401, 97–106. [Google Scholar] [CrossRef]
- Sharghi, H.; Ebrahimpourmoghaddam, S.; Doroodmand, M.M.; Purkhosrow, A. Synthesis of Vasorelaxaing 1,4-Disubstituted 1,2,3-Triazoles Catalyzed by a 4′-Phenyl-2,2′:6′,2′′-Terpyridine Copper(II) Complex Immobilized on Activated Multiwalled Carbon Nanotubes. Asian J. Org. Chem. 2012, 1, 377–388. [Google Scholar] [CrossRef]
- Carraher, C.E.; Culbertson, B.M.; Pittman, C.U.; Sheats, J.E.; Zeldin, M. Meeting Metal-Containing Polymeric Materials; American Chemical Society: Washington, DC, USA, 1996; ISBN 978-1-4613-0365-7. [Google Scholar]
- Abramova, L.I.; Bayburdov, T.A.; Grigoryan, E.P. Polyacrylamide. Kurenkov, V.F., Ed.; Chemistry: Moscow, Russia, 1992; ISBN 5-7245-0684-X. [Google Scholar]
- Choi, Y.; Park, K.; Choi, H.; Son, D.; Shin, M. Self-Healing, Stretchable, Biocompatible, and Conductive Alginate Hydrogels through Dynamic Covalent Bonds for Implantable Electronics. Polymers 2021, 13, 1133. [Google Scholar] [CrossRef]
- Hrib, J.; Chylikova Krumbholcova, E.; Duskova-Smrckova, M.; Hobzova, R.; Sirc, J.; Hruby, M.; Michalek, J.; Hodan, J.; Lesny, P.; Smucler, R. Hydrogel Tissue Expanders for Stomatology. Part II. Poly(Styrene-Maleic Anhydride) Hydrogels. Polymers 2019, 11, 1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; An, H.; Xi, B.; Yang, Y.; Qin, J.; Wang, Y.; He, Y.; Wang, X. Self-Healing Hydrogels with both LCST and UCST through Cross-Linking Induced Thermo-Response. Polymers 2019, 11, 490. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, Strong, and Tough Hydrogels with Good Chemical Stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Y.; Ding, H.; Qian, J.; Yin, J.; Wu, Z.L.; Song, Y.; Zheng, Q. Metal-Coordination Complexes Mediated Physical Hydrogels with High Toughness, Stick–Slip Tearing Behavior, and Good Processability. Macromolecules 2016, 49, 9637–9646. [Google Scholar] [CrossRef]














| Concentration (wt.%) | AM/AA/MTpy (%/%/%) | ||||||
|---|---|---|---|---|---|---|---|
| Samples | Water | AA | AM | KPS | MCM1 | MCM2 | |
| Gel 1 | 80 | 17 | 3 | 0.2 | - | - | 15/85 |
| Gel 2 | 80 | 10 | 10 | 0.2 | - | - | 50/50 |
| Gel 3 | 80 | 3 | 17 | 0.2 | - | - | 85/15 |
| Gel 4 | 50 | 25 | 25 | 0.2 | - | - | 50/50 |
| Gel 5 | 80 | 2.85 | 16.9 | 0.2 | - | 0.25 | 84.5/14.25/1.25 |
| Gel 6 | 80 | 2.85 | 16.9 | 0.2 | 0.25 | - | 84.5/14.25/1.25 |
| Gel 7 | 80 | 2.9 | 16.9 | 0.2 | - | 0.2 | 84.5/14.5/1 |
| Gel 8 | 80 | 2.9 | 16.9 | 0.2 | 0.2 | - | 84.5/14.5/1 |
| Gel 9 | 80 | 9.9 | 9.9 | 0.2 | - | 0.2 | 49.5/49.5/1 |
| Gel 10 | 80 | 9.9 | 9.9 | 0.2 | 0.2 | - | 49.5/49.5/1 |
| Gel 11 | 80 | 16.9 | 2.9 | 0.2 | - | 0.2 | 14.5/84.5/1 |
| Gel 12 | 80 | 16.9 | 2.9 | 0.2 | 0.2 | - | 14.5/84.5/1 |
| Gel 13 | 50 | 24.9 | 24.9 | 0.2 | - | 0.2 | 49.5/49.5/1 |
| Gel 14 | 50 | 24.9 | 24.9 | 0.2 | 0.2 | - | 49.5/49.5/1 |
| Sample | Elemental content (wt.%) (Found/Calculated) | AM/AA/MCM (%/%/%) | AM/AA/MCM (%/%/%) | |||
|---|---|---|---|---|---|---|
| C | H | N | M | Calculated | Found | |
| Copolymer 5 | 41.77 | 7.61 | 13.54 | 0.28/0.25 | 84.5/14.25/1.25 | 59.7/38.9/1.4 |
| Copolymer 6 | 42.58 | 7.52 | 14.27 | 0.21/0.17 | 84.5/14.25/1.25 | 62.3/37.7/1.1 |
| Copolymer 7 | 40.97 | 7.46 | 13.52 | 0.09/0.2 | 84.5/14.5/1 | 63.0/36.3/0.7 |
| Copolymer 8 | 42.17 | 7.49 | 13.50 | 0.11/0.14 | 84.5/14.5/1 | 59.5/39.5/1 |
| Copolymer 9 | 44.03 | 6.99 | 8.65 | 0.09/0.14 | 49.5/49.5/1 | 39.6/59.8/0.6 |
| Copolymer 10 | 43.83 | 7.05 | 8.82 | 0.15/0.1 | 49.5/49.5/1 | 39.6/59.8/0.6 |
| № | Sample | TGA data | Tg (°C) (ASTM) | Residue at 550 °C (wt.%) | |||
|---|---|---|---|---|---|---|---|
| T5% (°C) | T10% (°C) | T20% (°C) | Tmax (°C) | ||||
| 1 | Copolymer 2 | 173 | 202 | 269 | 217; 382 | 83 | 16.1 |
| 2 | Copolymer 6 | 120 | 218 | 275 | 217; 378 | 88 | 19.5 |
| 3 | Copolymer 7 | 117 | 185 | 253 | 222; 382 | 89 | 23.8 |
| 4 | Copolymer 8 | 71 | 120 | 228 | 221; 283; 383 | 89 | 23.4 |
| 5 | Copolymer 9 | 124 | 177 | 220 | 210; 381 | 78 | 3.4 |
| 6 | Copolymer 10 | 116 | 175 | 235 | 220; 373 | 80 | 16.2 |
| 7 | Copolymer 12 | 198 | 262 | 324 | 200; 278; 379 | 68 | 21.3 |
| 8 | Copolymer 14 | 177 | 208 | 330 | 180; 382 | 67 | 33.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baimuratova, R.K.; Dzhardimalieva, G.I.; Vaganov, E.V.; Lesnichaya, V.A.; Kugabaeva, G.D.; Kydralieva, K.A.; Zhinzhilo, V.A.; Uflyand, I.E. Novel Self-Healing Metallocopolymers with Pendent 4-Phenyl-2,2′:6′,2″-terpyridine Ligand: Kinetic Studies and Mechanical Properties. Polymers 2021, 13, 1760. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111760
Baimuratova RK, Dzhardimalieva GI, Vaganov EV, Lesnichaya VA, Kugabaeva GD, Kydralieva KA, Zhinzhilo VA, Uflyand IE. Novel Self-Healing Metallocopolymers with Pendent 4-Phenyl-2,2′:6′,2″-terpyridine Ligand: Kinetic Studies and Mechanical Properties. Polymers. 2021; 13(11):1760. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111760
Chicago/Turabian StyleBaimuratova, Rose K., Gulzhian I. Dzhardimalieva, Evgeniy V. Vaganov, Valentina A. Lesnichaya, Gulsara D. Kugabaeva, Kamila A. Kydralieva, Vladimir A. Zhinzhilo, and Igor E. Uflyand. 2021. "Novel Self-Healing Metallocopolymers with Pendent 4-Phenyl-2,2′:6′,2″-terpyridine Ligand: Kinetic Studies and Mechanical Properties" Polymers 13, no. 11: 1760. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111760