Enhancing Pervaporation Membrane Selectivity by Incorporating Star Macromolecules Modified with Ionic Liquid for Intensification of Lactic Acid Dehydration
Abstract
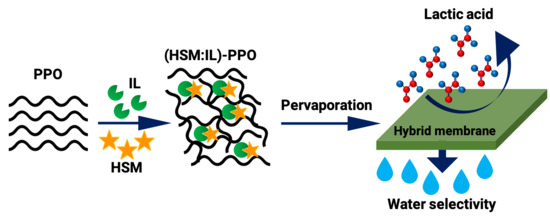
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Computational Methods
2.4. Membrane Characterization
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. FTIR Analysis
2.4.3. X-ray Diffraction (XRD)
2.4.4. Sorption Study
2.5. Pervaporation
3. Results
3.1. Membrane Structure Characterization
3.2. Transport Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robeson, L.M. Polymer Membranes. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 325–347. [Google Scholar]
- Vandezande, P. Next-Generation Pervaporation Membranes: Recent Trends, Challenges and Perspectives. In Pervaporation, Vapour Permeation and Membrane Distillation: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 107–141. ISBN 9781782422563. [Google Scholar]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Ermakov, S.; Roizard, D.; Penkova, A. Enhanced Pervaporation Properties of PVA-Based Membranes Modified with Polyelectrolytes. Application to IPA Dehydration. Polymers 2019, 12, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Mazur, A.; Lahderanta, E.; Ermakov, S.; Penkova, A. Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol. Polymers 2020, 12, 864. [Google Scholar] [CrossRef]
- Polotskaya, G.; Pulyalina, A.; Goikhman, M.; Podeshvo, I.; Rostovtseva, V.; Shugurov, S.; Gofman, I.; Saprykina, N.; Gulii, N.; Loretsyan, N.; et al. Novel Polyheteroarylene Membranes for Separation of Methanol-Hexane Mixture by Pervaporation. Sci. Rep. 2018, 8, 17849. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Xie, Z.; Cran, M.; Wu, C.; Gray, S. Dimensional Nanofillers in Mixed Matrix Membranes for Pervaporation Separations: A Review. Membranes 2020, 10, 193. [Google Scholar] [CrossRef]
- Salehi, E.; Heidary, F.; Daraei, P.; Keyhani, M.; Behjomanesh, M. Carbon nanostructures for advanced nanocomposite mixed matrix membranes: A comprehensive overview. Rev. Chem. Eng. 2020, 36, 723–748. [Google Scholar] [CrossRef]
- Livi, S.; Gérard, J.-F.; Duchet-Rumeau, J. Ionic Liquids as Polymer Additives. In Applications of Ionic Liquids in Polymer Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–21. [Google Scholar]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2018, 34, 341–363. [Google Scholar] [CrossRef]
- Kárászová, M.; Kacirková, M.; Friess, K.; Izák, P. Progress in separation of gases by permeation and liquids by pervaporation using ionic liquids: A review. Sep. Purif. Technol. 2014, 132, 93–101. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, C.; Avilés, M.-D.; Pamies, R.; Carrión-Vilches, F.-J.; Sanes, J.; Bermúdez, M.-D. Extruded PLA Nanocomposites Modified by Graphene Oxide and Ionic Liquid. Polymers 2021, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- O’Harra, K.E.; DeVriese, E.M.; Turflinger, E.M.; Noll, D.M.; Bara, J.E. Design and Gas Separation Performance of Imidazolium Poly(ILs) Containing Multivalent Imidazolium Fillers and Crosslinking Agents. Polymers 2021, 13, 1388. [Google Scholar] [CrossRef] [PubMed]
- Izák, P.; Ruth, W.; Fei, Z.; Dyson, P.J.; Kragl, U. Selective removal of acetone and butan-1-ol from water with supported ionic liquid–polydimethylsiloxane membrane by pervaporation. Chem. Eng. J. 2008, 139, 318–321. [Google Scholar] [CrossRef]
- Izák, P.; Friess, K.; Hynek, V.; Ruth, W.; Fei, Z.; Dyson, J.P.; Kragl, U. Separation properties of supported ionic liquid–polydimethylsiloxane membrane in pervaporation process. Desalination 2009, 241, 182–187. [Google Scholar] [CrossRef]
- Mai, N.L.; Kim, S.H.; Ha, S.H.; Shin, H.S.; Koo, Y.-M. Selective recovery of acetone-butanol-ethanol from aqueous mixture by pervaporation using immobilized ionic liquid polydimethylsiloxane membrane. Korean J. Chem. Eng. 2013, 30, 1804–1809. [Google Scholar] [CrossRef]
- Heitmann, S.; Krings, J.; Kreis, P.; Lennert, A.; Pitner, W.R.; Górak, A.; Schulte, M.M. Recovery of n-butanol using ionic liquid-based pervaporation membranes. Sep. Purif. Technol. 2012, 97, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Uragami, T.; Matsuoka, Y.; Miyata, T. Permeation and separation characteristics in removal of dilute volatile organic compounds from aqueous solutions through copolymer membranes consisted of poly(styrene) and poly(dimethylsiloxane) containing a hydrophobic ionic liquid by pervaporation. J. Memb. Sci. 2016, 506, 109–118. [Google Scholar] [CrossRef]
- Izák, P.; Köckerling, M.; Kragl, U. Solute transport from aqueous mixture throught supported ionic liquid membrane by pervaporation. Desalination 2006, 199, 96–98. [Google Scholar] [CrossRef]
- Ajala, E.O.; Olonade, Y.O.; Ajala, M.A.; Akinpelu, G.S. Lactic Acid Production from Lignocellulose—A Review of Major Challenges and Selected Solutions. ChemBioEng Rev. 2020, 7, 38–49. [Google Scholar] [CrossRef]
- Jantasee, S.; Kienberger, M.; Mungma, N.; Siebenhofer, M. Potential and assessment of lactic acid production and isolation—A review. J. Chem. Technol. Biotechnol. 2017, 92, 2885–2893. [Google Scholar] [CrossRef]
- Plackett, D. Biopolymers—New Materials for Sustainable Films and Coatings; John Wiley & Sons: Chichester, UK, 2011; ISBN 9781119994312. [Google Scholar]
- Vu, D.T.; Kolah, A.K.; Asthana, N.S.; Peereboom, L.; Lira, C.T.; Miller, D.J. Oligomer distribution in concentrated lactic acid solutions. Fluid Phase Equilib. 2005, 236, 125–135. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Q.; Zhao, W.; Ma, H.; Sakata, K. Extraction and purification of lactic acid from fermentation broth by esterification and hydrolysis method. Sep. Purif. Technol. 2006, 49, 43–48. [Google Scholar] [CrossRef]
- Lipnizki, F.; Field, R.W.; Ten, P.-K. Pervaporation-based hybrid process: A review of process design, applications and economics. J. Memb. Sci. 1999, 153, 183–210. [Google Scholar] [CrossRef]
- Delgado, P.; Sanz, M.T.; Beltrán, S.; Núñez, L.A. Ethyl lactate production via esterification of lactic acid with ethanol combined with pervaporation. Chem. Eng. J. 2010, 165, 693–700. [Google Scholar] [CrossRef]
- Khunnonkwao, P.; Boontawan, P.; Haltrich, D.; Maischberger, T.; Boontawan, A. Purification of l-(+)-lactic acid from pre-treated fermentation broth using vapor permeation-assisted esterification. Process Biochem. 2012, 47, 1948–1956. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Pinho, S.P.; Rodrigues, A.E. Batch and continuous studies for ethyl lactate synthesis in a pervaporation membrane reactor. J. Memb. Sci. 2010, 361, 43–55. [Google Scholar] [CrossRef]
- Wasewar, K.; Patidar, S.; Agarwal, V.K. Esterification of lactic acid with ethanol in a pervaporation reactor: Modeling and performance study. Desalination 2009, 243, 305–313. [Google Scholar] [CrossRef]
- Rathod, A.P.; Wasewar, K.L.; Sonawane, S.S. Intensification of esterification reaction of lactic acid with iso-propanol using pervaporation reactor. Procedia Eng. 2013, 51, 456–460. [Google Scholar] [CrossRef] [Green Version]
- Benedict, D.J.; Parulekar, S.J.; Tsai, S.P. Esterification of lactic acid and ethanol with/without pervaporation. Ind. Eng. Chem. Res. 2003, 42, 2282–2291. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hasegawa, W.; Kondo, K.; Shimamura, T.; Tsuji, M. Application of supported ionic liquid membranes using a flat sheet and hollow fibers to lactic acid recovery. Desalin. Water Treat. 2010, 14, 37–46. [Google Scholar] [CrossRef]
- Matsumoto, M.; Panigrahi, A.; Murakami, Y.; Kondo, K. Effect of ammonium- and phosphonium-based ionic liquids on the separation of lactic acid by supported ionic liquid membranes (SILMs). Membranes 2011, 1, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Murakami, Y.; Minamidate, Y.; Kondo, K. Separation of Lactic Acid through Polymer Inclusion Membranes Containing Ionic Liquids. Sep. Sci. Technol. 2012, 47, 354–359. [Google Scholar] [CrossRef]
- Marták, J.; Schlosser, Š.; Vlčková, S. Pertraction of lactic acid through supported liquid membranes containing phosphonium ionic liquid. J. Memb. Sci. 2008, 318, 298–310. [Google Scholar] [CrossRef]
- Duke, M.C.; Lim, A.; Nielsen, L. Temperature and durability studies of lactic acid dehydration with inorganic membranes. In Proceedings of the 2006 International Conference on Nanoscience and Nanotechnology (ICONN), Brisbane, QLD, Australia, 3–7 July 2006; Australian Research Council: Canberra, Australia, 2006; pp. 520–523. [Google Scholar] [CrossRef]
- Delgado, P.; Sanz, M.T.; Beltrán, S. Pervaporation study for different binary mixtures in the esterification system of lactic acid with ethanol. Sep. Purif. Technol. 2008, 64, 78–87. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Lebedev, V.T.; Pulyalina, A.Y.; Vinogradova, L.V. Structure and transport properties of pervaporation membranes based on polyphenylene oxide and heteroarm star polymers. Pet. Chem. 2016, 56, 920–930. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Pulyalina, A.Y.; Rostovtseva, V.A.; Toikka, A.M.; Saprykina, N.N.; Vinogradova, L. V Effect of polystyrene stars with fullerene C60 cores on pervaporation properties of poly(phenylene oxide) membrane. Polym. Int. 2016, 65, 407–414. [Google Scholar] [CrossRef]
- Rostovtseva, V.; Pulyalina, A.; Rudakova, D.; Vinogradova, L.; Polotskaya, G. Strongly Selective Polymer Membranes Modified with Heteroarm Stars for the Ethylene Glycol Dehydration by Pervaporation. Membranes 2020, 10, 86. [Google Scholar] [CrossRef]
- Pulyalina, A.; Porotnikov, D.; Rudakova, D.; Faykov, I.; Chislova, I.; Rostovtseva, V.; Vinogradova, L.; Toikka, A.; Polotskaya, G. Advanced membranes containing star macromolecules with C 60 core for intensification of propyl acetate production. Chem. Eng. Res. Des. 2018, 135, 197–206. [Google Scholar] [CrossRef]
- Khayet, M.; Villaluenga, J.P.G.; Godino, M.P.; Mengual, J.I.; Seoane, B.; Khulbe, K.C.; Matsuura, T. Preparation and application of dense poly(phenylene oxide) membranes in pervaporation. J. Colloid Interface Sci. 2004, 278, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Polotskaya, G.A.; Penkova, A.V.; Toikka, A.M. Fullerene-containing polyphenylene oxide membranes for pervaporation. Desalination 2006, 200, 400–402. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Krasnopeeva, E.L.; Kalyuzhnaya, L.M.; Saprykina, N.N.; Vinogradova, L.V. Mixed matrix membranes with hybrid star-shaped macromolecules for mono- and dihydric alcohols pervaporation. Sep. Purif. Technol. 2015, 143, 192–200. [Google Scholar] [CrossRef]
- Vinogradova, L.V. Star-shaped polymers with the fullerene C60 branching center. Russ. Chem. Bull. 2012, 61, 907–925. [Google Scholar] [CrossRef]
- Sure, R.; Grimme, S. Corrected small basis set Hartree-Fock method for large systems. J. Comput. Chem. 2013, 34, 1672–1685. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Pulyalina, A.; Tataurov, M.; Faykov, I.; Rostovtseva, V.; Polotskaya, G. Polyimide Asymmetric Membrane vs. Dense Film for Purification of MTBE Oxygenate by Pervaporation. Symmetry 2020, 12, 436. [Google Scholar] [CrossRef] [Green Version]
- Pulyalina, A.; Toikka, A.; Polotskaya, G. Investigation of pervaporation membranes based on polycarbamide: Effect of residual solvent. Pet. Chem. 2014, 54, 573–579. [Google Scholar] [CrossRef]
- Pulyalina, A.; Polotskaya, G.; Kalyuzhnaya, L.; Sushchenko, I.; Meleshko, T.; Yakimanskii, A.; Chislov, M.; Toikka, A. Sorption and transport of aqueous isopropanol solutions in polyimide-poly(aniline-co-anthranilic acid) composites. Russ. J. Appl. Chem. 2011, 84, 840–846. [Google Scholar] [CrossRef]
- Pulyalina, A.; Rostovtseva, V.; Polotskaya, G.; Vinogradova, L.; Zoolshoev, Z.; Simonova, M.; Hairullin, A.; Toikka, A.; Pientka, Z. Hybrid macromolecular stars incorporated poly(phenylene oxide) membranes: Organization, physical, and gas separation properties. Polymers 2019, 172, 355–364. [Google Scholar] [CrossRef]
- Lasseuguette, E.; McClements, J.; Koutsos, V.; Schäfer, T.; Ferrari, M.C. Ionic liquid mediated surface micropatterning of polymer blends. J. Appl. Polym. Sci. 2018, 135, 2–9. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Barton, A.F.M. CRC Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781351457194. [Google Scholar]
- Polotskaya, G.; Pulyalina, A.; Lebedev, V.; Török, G.; Rudakova, D.; Vinogradova, L. Novel view at hybrid membranes containing star macromolecules using neutron scattering and pervaporation dehydration of acetic acid. Mater. Des. 2020, 186, 108352. [Google Scholar] [CrossRef]












| Liquid | Molecular Weight (g/mol) | Molar Volume (cm3/mol) | Radius of Gyration (Å) [24] | Density (g/cm3) (20°) | Vapor Pressure (bar) [37] | Hildebrand Solubility Parameter δ, (J/cm3)1/2 |
|---|---|---|---|---|---|---|
| Water | 18.0 | 18.83 | 0.615 | 0.997 | 1.24 × 10−1 | 49.6 |
| Lactic acid | 90.08 | 88.98 | 3.298 | 1.225 | 1.08 × 10−3 | 34.1 |
| Membrane | T, K | Water in Feed, wt.% | Total Flux, kg/m2 h | Water in Permeate, wt.% | PSI, kg/m2 h | Ref. |
|---|---|---|---|---|---|---|
| Pervap 2201 | 327 | 40 | 0.8 | 96.0 | 28.8 | [37] |
| (HSM:IL)-PPO | 293 | 40 | 0.03 | 99.8 | 31.9 | Present work |
| (HSM:IL)-PPO/UPM | 293 | 40 | 0.06 | 99.8 | 65.8 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostovtseva, V.; Pulyalina, A.; Dubovenko, R.; Faykov, I.; Subbotina, K.; Saprykina, N.; Novikov, A.; Vinogradova, L.; Polotskaya, G. Enhancing Pervaporation Membrane Selectivity by Incorporating Star Macromolecules Modified with Ionic Liquid for Intensification of Lactic Acid Dehydration. Polymers 2021, 13, 1811. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111811
Rostovtseva V, Pulyalina A, Dubovenko R, Faykov I, Subbotina K, Saprykina N, Novikov A, Vinogradova L, Polotskaya G. Enhancing Pervaporation Membrane Selectivity by Incorporating Star Macromolecules Modified with Ionic Liquid for Intensification of Lactic Acid Dehydration. Polymers. 2021; 13(11):1811. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111811
Chicago/Turabian StyleRostovtseva, Valeriia, Alexandra Pulyalina, Roman Dubovenko, Ilya Faykov, Kseniya Subbotina, Natalia Saprykina, Alexander Novikov, Ludmila Vinogradova, and Galina Polotskaya. 2021. "Enhancing Pervaporation Membrane Selectivity by Incorporating Star Macromolecules Modified with Ionic Liquid for Intensification of Lactic Acid Dehydration" Polymers 13, no. 11: 1811. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111811