Non-Woven Fabrics Based on Nanocomposite Nylon 6/ZnO Obtained by Ultrasound-Assisted Extrusion for Improved Antimicrobial and Adsorption Methylene Blue Dye Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Nylon 6/ZnO Nanocomposites
2.2. Synthesis of Non-Woven Fabrics
2.3. Characterization
Adsorption Isotherm
2.4. Nonwoven Fabric Regeneration
3. Results and Discussion
3.1. Microscopy Characterization of Nylon 6 and Nanocomposites
3.2. Mechanical Properties
3.3. Antimicrobial Properties
3.4. Methylene Blue Adsorption
3.4.1. Effect of UV Radiation on MB Adsorption
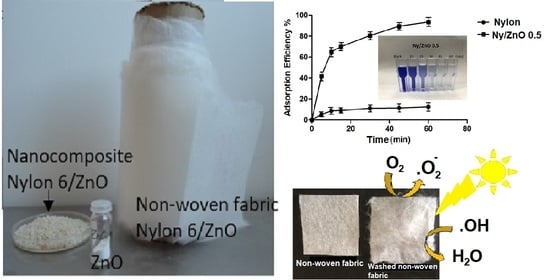
3.4.2. Adsorption Efficiency of MB as a Function of Time
3.4.3. Adsorption Isotherm
3.4.4. Effect of pH on the Adsorption MB
3.4.5. Mechanism of the Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drumond-Chequer, F.M.; de Oliveira, G.A.R.; Anastácio-Ferraz, E.R.; Carvalho-Cardoso, J.; Zanoni, M.V.B.; de Oliveira, D.P. Textile dyes: Dyeing process and environmental impact. Eco-Friendly Text. Dye. Finish. 2013, 6, 151–176. [Google Scholar]
- Cusioli, L.F.; Quesada, H.B.; Baptista, A.T.; Gomes, R.G.; Bergamasco, R. Soybean hulls as a low-cost biosorbent for removal of methylene blue contaminant. Environ. Prog. Sustain. Energy 2020, 39, e13328. [Google Scholar] [CrossRef]
- Pelaseyed, S.S.; Hosseini, H.R.M.; Nokhbedehghan, Z.; Samadikuchaksaraei, A. PLGA/TiO2 nanocomposite scaffolds for biomedical applications: Fabrication, photocatalytic, and antibacterial properties. BioImpacts BI 2021, 11, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Arefpour, A.; Baghbaderani, M.Z.; Shafieirad, A.; Kasiri-Asgarani, M.; Monshi, A.; Karbasi, S.; Goldanlou, A.S. Mechanical behaviour, hybridisation and osteoblast activities of novel baghdadite/PCL-graphene nanocomposite scaffold: Viability, cytotoxicity and calcium activity. Mater. Technol. 2021, 1–15. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Shahat, A.; Khan, Z.A.; Alshitari, W.; Guibal, E. Magnetic metal oxide-organic framework material for ultrasonic-assisted sorption of titan yellow and rose bengal from aqueous solutions. Chem. Eng. J. 2020, 392, 123635. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. Multifunctional eco-friendly sorbent based on marine brown algae and bivalve shells for subsequent uptake of Congo red dye and copper (II) ions. J. Environ. Chem. Eng. 2020, 8, 103915. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Elgarahy, A.M.; Khan, Z.A.; Almughamisi, M.S.; Al-Bogami, A.S. Perspectives regarding metal/mineral-incorporating materials for water purification: With special focus on Cr (vi) removal. Mater. Adv. 2020, 1, 1546–1574. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Elgarahy, A.M.; Mohammad, S.H. Use of beach bivalve shells located at Port Said coast (Egypt) as a green approach for methylene blue removal. J. Environ. Chem. Eng. 2017, 5, 578–587. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Elgarahy, A.M.; Elshoubaky, G.A.; Mohammad, S.H. Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells. J. Environ. Health Sci. Eng. 2020, 18, 35–50. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Elwakeel, K.Z.; Elshoubaky, G.A.; Mohammad, S.H. Microwave-accelerated sorption of cationic dyes onto green marine algal biomass. Environ. Sci. Pollut. Res. 2019, 26, 22704–22722. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Natali, S. Magnetically modified agro-industrial wastes as efficient and easily recoverable adsorbents for water treatment. Chem. Eng. 2014, 38. [Google Scholar] [CrossRef]
- Asghar, H.M.A.; Hussain, S.N.; Brown, N.W.; Roberts, E.P.L. Comparative adsorption–regeneration performance for newly developed carbonaceous adsorbent. J. Ind. Eng. Chem. 2019, 69, 90–98. [Google Scholar] [CrossRef]
- Kumlien, A.C.A.; Borrego, C.M.; Balcázar, J.L. Antimicrobial Resistance and Bacteriophages: An Overlooked Intersection in Water Disinfection. Trends Microbiol. 2021, 29, 517–527. [Google Scholar] [CrossRef]
- Hu, J.; Jahid, M.A.; Harish Kumar, N.; Harun, V. Fundamentals of the Fibrous Materials. In Handbook of Fibrous Materials; Hu, J., Kumar, B., Lu, J., Eds.; Wiley: Weinheim, Germany, 2020; pp. 1–36. [Google Scholar]
- Singh, G.; Joyce, E.M.; Beddow, J.; Mason, T.J. Evaluation of Antibacterial Activity of ZnO Nanoparticles Coated Sonochemically onto Textile Fabrics. J. Microbiol. Biotechnol. Food Sci. 2012, 2, 106–120. Available online: https://www.jmbfs.org/wp-content/uploads/2012/07/jmbfs-0117-singh.pdf (accessed on 24 March 2021).
- Saleem, S.; Qurashi, A.; Ahmad, F. ZnO nanostructures based biosensors for cancer and infectious disease applications: Perspectives, prospects and promises. Trends Anal. Chem. 2017, 86, 1–13. [Google Scholar]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. Vitr. 2009, 23, 1116–1122. [Google Scholar] [CrossRef]
- Leung, Y.H.; Xu, X.; Ma, A.P.; Liu, F.; Ng, A.M.; Shen, Z.; Gethings, L.A.; Guo, M.Y.; Djurišić, A.B.; Leung, F.C.; et al. Toxicity of ZnO and TiO2 to Escherichia coli cells. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.A. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 2020, 15, 1–19. [Google Scholar] [CrossRef]
- Wehmann, M.; McCulloch, W.J.G. Melt blowing technology. In Polypropylene; Springer: Dordrecht, The Netherlands, 1999; pp. 415–420. [Google Scholar]
- Verbič, A.; Gorjanc, M.; Simončič, B. Zinc oxide for functional textile coatings: Recent advances. Coatings 2019, 9, 550. [Google Scholar] [CrossRef] [Green Version]
- Ávila-Orta, C.A.; Colunga, J.G.M.; Baquéz, D.B.; López, C.E.R.; Delgado, V.J.C.; Morones, P.G.; González, J.A.R. U.S. Patent Application No. 13/258,930, 2012. Available online: https://patents.google.com/patent/US20120098163A1/en?oq=13%2f258%2c930 (accessed on 26 April 2012).
- Shinde, V.V.; Jadhav, P.R.; Kim, J.H.; Patil, P.S. One-step synthesis and characterization of anisotropic silver nanoparticles: Application for enhanced antibacterial activity of natural fabric. J. Mater. Sci. 2013, 48, 8393–8401. [Google Scholar] [CrossRef]
- Raza, Z.A.; Anwar, F.; Ahmad, S.; Aslam, M. Fabrication of ZnO incorporated chitosan nanocomposites for enhanced functional properties of cellulosic fabric. Mater. Res. Express. 2016, 3, 115001. [Google Scholar] [CrossRef]
- Zheng, J.; Ozisik, R.; Siegel, R.W. Disruption of self-assembly and altered mechanical behavior in polyurethane/zinc oxide nanocomposites. Polymer 2005, 46, 10873–10882. [Google Scholar] [CrossRef]
- Mowery, B.P.; Lindner, A.H.; Weisblum, B.; Stahl, S.S.; Gellman, S.H. Structure-activity relationships among random nylon-3 copolymers that mimic antibacterial host-defense peptides. J. Am. Chem. Soc. 2009, 131, 9735–9745. [Google Scholar] [CrossRef]
- Janaki, A.C.; Sailatha, E.; Gunasekaran, S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 144, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.A.; Anwar, F. Impregnation of zinc oxide mediated chitosan nano-composites on polyester fabric for performance characteristics. Fibers Polym. 2016, 17, 1378–1383. [Google Scholar] [CrossRef]
- Erem, A.D.; Ozcan, G.; Skrifvars, M. Antibacterial activity of PA6/ZnO nanocomposite fibers. Text. Res. J. 2011, 81, 1638–1646. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, D.Y.; Mali, M.G.; Al-Deyab, S.S.; Swihart, M.T.; Yoon, S.S. Supersonically blown nylon-6 nanofibers entangled with graphene flakes for water purification. Nanoscale 2015, 7, 19027–19035. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pechyen, C. Role of ZnO on nylon 6 surface and the photocatalytic efficiency of methylene blue for wastewater treatment. Colloid Polym. Sci. 2016, 294, 1217–1224. [Google Scholar] [CrossRef]
- Lam, S.M.; Kee, M.W.; Sin, J.C. Influence of PVP surfactant on the morphology and properties of ZnO micro/nanoflowers for dye mixtures and textile wastewater degradation. Mater. Chem. Phys. 2018, 212, 35–43. [Google Scholar] [CrossRef]
- Ashraf, M.; Champagne, P.; Perwuelz, A.; Campagne, C.; Leriche, A. Photocatalytic solution discoloration and self-cleaning by polyester fabric functionalized with ZnO nanorods. J. Ind. Text. 2015, 44, 884–898. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Ávila-Orta, C.A.; Cadenas-Pliego, G.; Cabello-Alvarado, C.; Pérez-Alvarez, M.; Reyes-Rodríguez, P.; Inam, F.; Cortés-Hernández, D.A.; Quiñones-Jurado, Z.V. Synthesis of Nylon 6/Modified Carbon Black Nanocomposites for Application in Uric Acid Adsorption. Materials 2020, 13, 5173. [Google Scholar] [CrossRef]
- Sierra, R.; Pérez, M.; Valdez, J.; Ávila, C.; Jimenez, E.J.; Mata, J.; Soto, E.; Cadenas, G. Synthesis and Thermomechanical Characterization of Nylon 6/Cu Nanocomposites Produced by an Ultrasound-Assisted Extrusion Method. Adv. Mater. Sci. Eng. 2018, 2018, 4792735. [Google Scholar]
- Jawad, A.H.; Abdulhameed, A.S. Statistical modeling of methylene blue dye adsorption by high surface area mesoporous activated carbon from bamboo chip using KOH-assisted thermal activation. Energy Ecol. Environ. 2020, 5, 456–469. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Wang, A. Enhanced adsorption of Methylene Blue from aqueous solution by chitosan-g-poly (acrylic acid)/vermiculite hydrogel composites. J. Environ. Sci. 2010, 22, 486–493. [Google Scholar] [CrossRef]
- Lavecchia, R.; Medici, F.; Patterer, S.; Zuorro, A. Lead removal from water by adsorption on spent coffee grounds. Chem. Eng. Trans. 2016, 47, 295–300. [Google Scholar]
- Elwakeel, K.Z.; Elgarahy, A.M.; Al-Bogami, A.S.; Hamza, M.F.; Guibal, E. 2-Mercaptobenzimidazole-functionalized chitosan for enhanced removal of methylene blue: Batch and column studies. J. Environ. Chem. Eng. 2021, 9, 105609. [Google Scholar] [CrossRef]
- Guan, K. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf. Coat. Technol. 2005, 191, 155–160. [Google Scholar] [CrossRef]












| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| k | qmax | R2 | n | KF | R2 | |
| Nylon | 1.20 | 196.92 | 0.9387 | 9.61 | 52.17 | 0.9817 |
| Ny/ZnO 0.5 | 0.023 | 0.33 | 0.9527 | 0.30 | 5.41 | 0.8346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade-Guel, M.; Ávila-Orta, C.A.; Cabello-Alvarado, C.; Cadenas-Pliego, G.; Esparza-González, S.C.; Pérez-Alvarez, M.; Quiñones-Jurado, Z.V. Non-Woven Fabrics Based on Nanocomposite Nylon 6/ZnO Obtained by Ultrasound-Assisted Extrusion for Improved Antimicrobial and Adsorption Methylene Blue Dye Properties. Polymers 2021, 13, 1888. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111888
Andrade-Guel M, Ávila-Orta CA, Cabello-Alvarado C, Cadenas-Pliego G, Esparza-González SC, Pérez-Alvarez M, Quiñones-Jurado ZV. Non-Woven Fabrics Based on Nanocomposite Nylon 6/ZnO Obtained by Ultrasound-Assisted Extrusion for Improved Antimicrobial and Adsorption Methylene Blue Dye Properties. Polymers. 2021; 13(11):1888. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111888
Chicago/Turabian StyleAndrade-Guel, M., C. A. Ávila-Orta, C. Cabello-Alvarado, G. Cadenas-Pliego, S. C. Esparza-González, M. Pérez-Alvarez, and Z. V. Quiñones-Jurado. 2021. "Non-Woven Fabrics Based on Nanocomposite Nylon 6/ZnO Obtained by Ultrasound-Assisted Extrusion for Improved Antimicrobial and Adsorption Methylene Blue Dye Properties" Polymers 13, no. 11: 1888. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111888