Low-Denaturazing Glucose Oxidase Immobilization onto Graphite Electrodes by Incubation in Chitosan Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentation
2.2. Apparatus and Electrodes
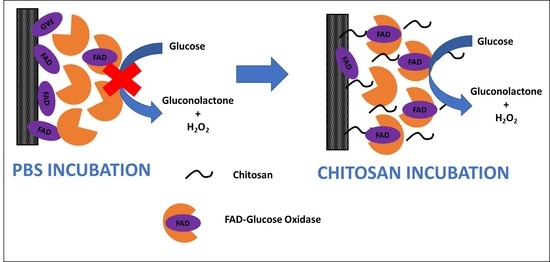
2.3. GOx Immobilization Procedure
2.4. Electrochemical Characterization
3. Results and Discussion
3.1. Bioelectrode Modification with Glucose Oxidase
3.2. Electrochemistry of Immobilized GOx in Chitosan Solutions
3.3. Evaluation of Immobilized GOx Activity
3.3.1. GOx Activity and Inhibition Assays
3.3.2. GOx Activity for Energy Harvesting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Clark, L.C., Jr. Electrochemical Device for Chemical Analysis. U.S. Patent 2913386A, 17 November 1959. [Google Scholar]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khor, S.M.; Choi, J.; Won, P.; Ko, S.H. Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes. Nanomaterials 2022, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Buaki-Sogó, M.; García-Carmona, L.; Gil-Agustí, M.; Zubizarreta, L.; García-Pellicer, M.; Quijano-López, A. Enzymatic Glucose-Based Bio-batteries: Bioenergy to Fuel Next-Generation Devices. Top. Curr. Chem. 2020, 378, 49. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Yin, Z.; Ji, Z.; Zhang, W.; Taylor, E.W.; Zeng, X.; Wei, J. The glucose effect on direct electrochemistry and electron transfer reaction of glucose oxidase entrapped in a carbon nanotube-polymer matrix. ChemistrySelect 2020, 5, 12224–12231. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Deng, X.; Li, J.; Huang, S.; Jin, X.; Zhu, X. Self-encapsulated enzyme through in situ growth of polypyrrole for high-performance enzymatic biofuel cell. Chem. Eng. J. 2021, 429, 132148. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Al-Lolage, F.A. There is no evidence to support literature claims of direct electron transfer (DET) for native glucose oxidase (GOx) at carbon nanotubes or graphene. J. Electroanal. Chem. 2018, 819, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [Green Version]
- You, C.; Li, X.; Zhang, S.; Kong, J.; Zhao, D.; Liu, B. Electrochemistry and biosensing of glucose oxidase immobilized on Pt-dispersed mesoporous carbon. Microchim. Acta 2009, 167, 109–116. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, B.; Gorton, L.; Ruzgas, T.; Jönsson, L.J. Characterization of graphite electrodes modified with laccase from Trametes Versicolor and their use for bioelectrochemical monitoring of phenolic compounds in flow injection analysis. Anal. Chim. Acta. 2003, 487, 3–14. [Google Scholar] [CrossRef]
- Jarosz-Wilkołazka, A.; Ruzgas, T.; Gorton, L. Use of laccase-modified electrode for amperometric detection of plant flavonoids. Enzyme Microb. Technol. 2004, 35, 238–241. [Google Scholar] [CrossRef]
- Onay, A.; Dogan, Ü.; Ciftci, H.; Cetin, D.; Suludere, Z.; Tamer, U. Amperometric glucose sensor based on the glucose oxidase enzyme immobilized on graphite rod electrode modified with Fe3O4-CS-Au magnetic nanoparticles. Ionics 2018, 24, 4015–4022. [Google Scholar] [CrossRef]
- Bandapati, M.; Krishnamurthy, B.; Goel, S. Fully Assembled Membraneless Glucose Biofuel Cell With MWCNT Modified Pencil Graphite Leads as Novel Bioelectrodes. IEEE Trans. NanoBioscience 2019, 18, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Chi, Q.; Zhang, J.; Dong, S.; Wang, E. Direct electrochemistry and surface characterization of glucose oxidase adsorbed on anodized carbon electrodes. Electrochimica Acta 1994, 39, 2431–2438. [Google Scholar] [CrossRef]
- Ianniello, R.M.; Lindsay, T.J.; Yacynych, A.M. Differential pulse voltammetric study of direct electron transfer in glucose oxidase chemically modified graphite electrodes. Anal. Chem. 1982, 54, 1098–1101. [Google Scholar] [CrossRef]
- Goran, J.M.; Mantilla, S.M.; Stevenson, K.J. Influence of Surface Adsorption on the Interfacial Electron Transfer of Flavin Adenine Dinucleotide and Glucose Oxidase at Carbon Nanotube and Nitrogen-Doped Carbon Nanotube Electrodes. Anal. Chem. 2013, 85, 1571–1581. [Google Scholar] [CrossRef]
- Pereira, S.; Santos, N.; Carvalho, A.; Fernandes, A.; Costa, F. Electrochemical Response of Glucose Oxidase Adsorbed on Laser-Induced Graphene. Nanomaterials 2021, 11, 1893. [Google Scholar] [CrossRef]
- Pilipenko, O.S.; Atyaksheva, L.F.; Poltorak, O.M.; Chukhrai, E.S. Dissociation and catalytic activity of oligomer forms of β-galactosidases. Russ. J. Phys. Chem. A 2007, 81, 990–994. [Google Scholar] [CrossRef]
- Poltorak, O.M.; Chukhrai, E.S.; Kozlenkov, A.A.; Chaplin, M.F.; Trevan, M.D. The putative common mechanism for inactivation of alkaline phosphatase isoenzymes. J. Mol. Catal. B Enzym. 1999, 7, 157–163. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Cava, F.; Mateo, C.; Rocha-Martín, J.; Guisán, J.M.; Berenguer, J.; Fernandez-Lafuente, R. Immobilization–stabilization of a new recombinant glutamate dehydrogenase from Thermus thermophilus. Appl. Microbiol. Biotechnol. 2008, 80, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, T.; Li, Y.; Kanada, N.; Ito, K.; Yoshimoto, T. Enhancement of the thermal stability of pyroglutamyl peptidase I by introduction of an intersubunit disulfide bond. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2001, 1547, 214–220. [Google Scholar] [CrossRef]
- Fuentes, M.; Segura, R.L.; Abian, O.; Betancor, L.; Hidalgo, A.; Mateo, C.; Fernandez-Lafuente, R.; Guisan, J.M. Determination of protein-protein interactions through aldehyde-dextran intermolecular cross-linking. Proteomics 2004, 4, 2602–2607. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Tsugawa, W.; Sode, K. Increased thermal stability of glucose dehydrogenase by cross-linking chemical modification. Biotechnol. Lett. 1999, 21, 199–202. [Google Scholar] [CrossRef]
- Tao, Q.; Li, A.; Zhang, Z.; Ma, R.; Shi, L. Stabilization of Multimeric Enzymes against Heat Inactivation by Chitosan-graft-poly(N-isopropylacrylamide) in Confined Spaces. ACS Biomater. Sci. Eng. 2017, 3, 3141–3145. [Google Scholar] [CrossRef]
- Dosadina, E.E.; Belov, A.A. Interaction Between Chitosan Solutions, Cellulose Carriers and Some of the Multi-enzyme Complexes. Int. J. Bioorg. Chem. 2017, 2, 51–60. [Google Scholar]
- Raza, Z.A.; Riaz, S.; Banat, I.M. Polyhydroxyalkanoates: Properties and chemical modification approaches for their functionalization. Biotechnol. Prog. 2018, 34, 29–41. [Google Scholar] [CrossRef]
- Riaz, S.; Rhee, K.Y.; Park, S.J. Polyhydroxyalkanoates (PHAs): Biopolymers for Biofuel and Biorefineries. Polymers 2021, 13, 253. [Google Scholar] [CrossRef]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Dabhade, A.; Jayaraman, S.; Paramasivan, B. Development of glucose oxidase-chitosan immobilized paper biosensor using screen-printed electrode for amperometric detection of Cr(VI) in water. 3 Biotech 2021, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- El Ichi, S.; Zebda, A.; Alcaraz, J.-P.; Laaroussi, A.; Boucher, F.; Boutonnat, J.; Reverdy-Bruas, N.; Chaussy, D.; Belgacem, M.N.; Cinquin, P.; et al. Bioelectrodes modified with chitosan for long-term energy supply from the body. Energy Environ. Sci. 2015, 8, 1017–1026. [Google Scholar] [CrossRef]
- Nasar, A.; Rahman, M.M. Applications of chitosan (CHI)-reduced graphene oxide (rGO)-polyaniline (PAni) conducting composite electrode for energy generation in glucose biofuel cell. Sci. Rep. 2020, 10, 10428. [Google Scholar]
- Rassas, I.; Braiek, M.; Bonhomme, A.; Bessueille, F.; Rafin, G.; Majdoub, H.; Jaffrezic-Renault, N. Voltammetric glucose biosensor based on glucose oxidase encapsulation in a chitosan-kappa-carrageenan polyelectrolyte complex. Mater. Sci. Eng. C 2019, 95, 152–159. [Google Scholar] [CrossRef]
- Bai, L.; Yuan, R.; Chai, Y.; Yuan, Y.; Wang, Y.; Xie, S. Direct electrochemistry and electrocatalysis of a glucose oxidase-functionalized bioconjugate as a trace label for ultrasensitive detection of thrombin. Chem. Commun. 2012, 48, 10972–10974. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, Y.; Shao, C.; Wan, L.; Jiao, K. Unadulterated Glucose Biosensor Based on Direct Electron Transfer of Glucose Oxidase Encapsulated Chitosan Modified Glassy Carbon Electrode. Electroanalysis 2008, 20, 520–526. [Google Scholar] [CrossRef]
- Kanda, M.; Brady, F.O.; Rajagopalan, K.V.; Handler, P. Studies on the Dissociation of Flavin Adenine Dinucleotide from Metalloflavoproteins. J. Biol. Chem. 1972, 247, 765–770. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Z.; Zhou, J. Hamiltonian replica exchange simulations of glucose oxidase adsorption on charged surfaces. Phys. Chem. Chem. Phys. 2018, 20, 14587–14596. [Google Scholar] [CrossRef]
- Gorton, L.; Johansson, G. Cyclic voltammetry of FAD adsorbed on graphite, glassy carbon, platinum, and gold electrodes. J. Electroanal. Chem. Inter. Electrochem. 1980, 113, 151–158. [Google Scholar] [CrossRef]
- Ianniello, R.M.; Lindsay, T.J.; Yacynych, A.M. Direct electron transfer in immobilized flavoenzyme chemically modified graphite electrodes. Anal. Chim. Acta 1982, 141, 23–32. [Google Scholar] [CrossRef]
- Coughlan, M.P.; Johnson, D.B. Preparation and properties of immobilised xanthine oxidase. Biochim. Biophys. Acta Enzymol. 1973, 302, 200–204. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Inter. Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Liang, B.; Guo, X.; Fang, L.; Hu, Y.; Yang, G.; Zhu, Q.; Wei, J.; Ye, X. Study of direct electron transfer and enzyme activity of glucose oxidase on graphene surface. Electrochem. Commun. 2015, 50, 1–5. [Google Scholar] [CrossRef]
- Kang, Z.; Jiao, K.; Yu, C.; Dong, J.; Peng, R.; Hu, Z.; Jiao, S. Direct electrochemistry and bioelectrocatalysis of glucose oxidase in CS/CNC film and its application in glucose biosensing and biofuel cells. RSC Adv. 2017, 7, 4572–4579. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, R.S. Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Vogt, S.; Schneider, M.; Schafer-Eberwin, H.; Noll, G. Determination of the pH dependent redox potential of glucose oxidase by spectroelectrochemistry. Anal. Chem. 2014, 86, 7530–7535. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Y. Direct electron transfer of glucose oxidase promoted by carbon nanotubes is without value in certain mediator-free applications. Microchim. Acta 2012, 176, 271–277. [Google Scholar] [CrossRef]
- Ivnitski, D.; Branch, B.; Atanassov, P.; Apblett, C. Glucose oxidase anode for biofuel cell based on direct electron transfer. Electrochem. Commun. 2006, 8, 1204–1210. [Google Scholar] [CrossRef]
- Gao, Y.-F.; Yang, T.; Yang, X.-L.; Zhang, Y.-S.; Xiao, B.-L.; Hong, J.; Sheibani, N.; Ghourchian, H.; Hong, T.; Moosavi-Movahedi, A.A. Direct electrochemistry of glucose oxidase and glucose biosensing on a hydroxyl fullerenes modified glassy carbon electrode. Biosens. Bioelectron. 2014, 60, 30–34. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, X.; Ma, W.; Yang, T.; Li, D.; Dai, B.; Zhang, Y. Direct electrochemistry of glucose oxidase based on one step electrodeposition of reduced graphene oxide incorporating polymerized l-lysine and its application in glucose sensing. Mater. Sci. Eng. C 2019, 104, 109880. [Google Scholar] [CrossRef] [PubMed]
- Dhekra, A.; Khaled, H.; Mina, S.; Mustapha, M.; Ali, O. Immobilization of Glucose Oxidase in Anthracene-Based Semi-Conducting Polymer: Application on Glucose Biosensing. J. Bioeng. Biomed. Sci. 2017, 7, 5. [Google Scholar]
- Wang, S.; Su, P.; Yang, Y. Online immobilized enzyme microreactor for the glucose oxidase enzymolysis and enzyme inhibition assay. Anal. Biochem. 2012, 427, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Rust, I.M.; Goran, J.M.; Stevenson, K.J. Amperometric Detection of Aqueous Silver Ions by Inhibition of Glucose Oxidase Immobilized on Nitrogen-Doped Carbon Nanotube Electrodes. Anal. Chem. 2015, 87, 7250–7257. [Google Scholar] [CrossRef]
- Yu, S.; Myung, N.V. Recent Advances in the Direct Electron Transfer-Enabled Enzymatic Fuel Cells. Front. Chem. 2021, 8, 620153. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buaki-Sogó, M.; García-Carmona, L.; Gil-Agustí, M.; García-Pellicer, M.; Quijano-López, A. Low-Denaturazing Glucose Oxidase Immobilization onto Graphite Electrodes by Incubation in Chitosan Solutions. Polysaccharides 2022, 3, 388-400. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3020023
Buaki-Sogó M, García-Carmona L, Gil-Agustí M, García-Pellicer M, Quijano-López A. Low-Denaturazing Glucose Oxidase Immobilization onto Graphite Electrodes by Incubation in Chitosan Solutions. Polysaccharides. 2022; 3(2):388-400. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3020023
Chicago/Turabian StyleBuaki-Sogó, Mireia, Laura García-Carmona, Mayte Gil-Agustí, Marta García-Pellicer, and Alfredo Quijano-López. 2022. "Low-Denaturazing Glucose Oxidase Immobilization onto Graphite Electrodes by Incubation in Chitosan Solutions" Polysaccharides 3, no. 2: 388-400. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides3020023