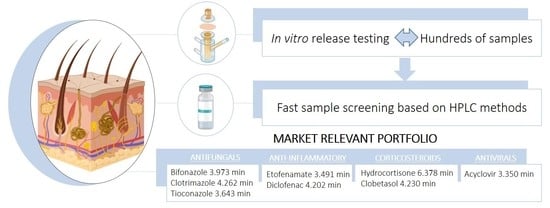
Fast Screening Methods for the Analysis of Topical Drug Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation and Chromatographic Conditions
2.3. Preparation of Stock Solutions, Calibration Standards, and Quality Controls
2.4. Method Validation
2.4.1. System Suitability
2.4.2. Limits of Detection and Quantification
2.4.3. Linearity
2.4.4. Accuracy and Precision
2.4.5. Ruggedness
2.4.6. Specificity and Selectivity
2.4.7. Stability
2.4.8. Method Applicability: In Vitro Release Testing
3. Results and Discussion
3.1. Method Validation
3.1.1. System Suitability
3.1.2. Limits of Detection and Quantification
3.1.3. Linearity
3.1.4. Accuracy and Precision
3.1.5. Ruggedness
3.1.6. Stability
3.2. Method Applicability: IVRT
3.2.1. In Silico Studies: Using Chemical Predictors to Support IVRT Development
3.2.2. IVRT
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACV | Acyclovir |
| API | Active pharmaceutical ingredient |
| BFZ | Bifonazole |
| CLB | Clobetasol |
| CLT | Clotrimazole |
| CQA | Critical quality attribute |
| DF | Diclofenac |
| ETF | Etofenamate |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| QC | Quality control |
| RP-HPLC | Reversed phase high performance liquid chromatography |
| TCZ | Tioconazole |
| TP | Topical product |
| USP | United States Pharmacopoeia |
References
- Mordor, I. Dermatological Therapeutics Market | Growth, Trends, and Forecast (2019–2024). Available online: https://www.mordorintelligence.com/industry-reports/dermatological-therapeutics-market (accessed on 29 October 2019).
- Patere, S.; Newman, B.; Wang, Y.; Choi, S.; Vora, S.; Ma, A.W.K.; Jay, M.; Lu, X. Influence of Manufacturing Process Variables on the Properties of Ophthalmic Ointments of Tobramycin. Pharm. Res. 2018, 35, 179. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.; Veiga, F.; Vitorino, C. Developing Cream Formulations: Renewed Interest in an Old Problem. J. Pharm. Sci. 2019, 108, 3240–3251. [Google Scholar] [CrossRef] [PubMed]
- Ethier, A.; Bansal, P.; Baxter, J.; Langley, N.; Richardson, N.; Patel, A.M. The Role of Excipients in the Microstructure of Topical Semisolid Drug Products. In The Role of Microstructure in Topical Drug Product Development; Langley, N., Michniak-Kohn, B., Osborne, D.W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 155–193. ISBN 978-3-030-17355-5. [Google Scholar]
- Murthy, S.N. Characterizing the Critical Quality Attributes and In Vitro Bioavailability of Acyclovir and Metronidazole Topical Products. In Proceedings of the FDA workshop on Bioequivalence Testing of Topical Drug Products, Silver Spring, MD, USA, 20 October 2019. [Google Scholar]
- Mangas-Sanjuán, V.; Pleguezuelos-Villa, M.; Merino-Sanjuán, M.; Hernández, M.J.; Nácher, A.; García-Arieta, A.; Peris, D.; Hidalgo, I.; Soler, L.; Sallan, M.; et al. Assessment of the inter-batch variability of microstructure parameters in topical semisolids and impact on the demonstration of equivalence. Pharmaceutics 2019, 11, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.; Veiga, F.; Figueiras, A.; Vitorino, C. A practical framework for implementing Quality by design to the development of topical drug products: Nanosystem-based dosage forms. Int. J. Pharm. 2018, 548, 385–399. [Google Scholar] [CrossRef]
- EMA. Draft Guideline on Quality and Equivalence of Topical Products; EMA: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Flühmann, B.; Ntai, I.; Borchard, G.; Simoens, S.; Mühlebach, S. Nanomedicines: The magic bullets reaching their target? Eur. J. Pharm. Sci. 2018, 128, 73–80. [Google Scholar] [CrossRef]
- Miranda, M.; Pais, A.A.; Cardoso, C.; Vitorino, C. aQbD as a platform for IVRT method development–A regulatory oriented approach. Int. J. Pharm. 2019, 572, 118695. [Google Scholar] [CrossRef]
- Shah, V.P.; Yacobi, A.; Ştefan Rădulescu, F.; Miron, D.S.; Lane, M. A science based approach to topical drug classification system (TCS). Int. J. Pharm. 2015, 491, 21–25. [Google Scholar] [CrossRef]
- Yacobi, A.; Shah, V.P.; Bashaw, E.; Benfeldt, E.; Davit, B.; Ganes, D.; Ghosh, T.; Kanfer, I.; Kasting, G.B.; Katz, L.; et al. Current Challenges in Bioequivalence, Quality, and Novel Assessment Technologies for Topical Products. Pharm. Res. 2014, 31, 837–846. [Google Scholar] [CrossRef]
- Tiffner, K.I.; Kanfer, I.; Augustin, T.; Raml, R.; Raney, S.G.; Sinner, F. A comprehensive approach to qualify and validate the essential parameters of an in vitro release test (IVRT) method for acyclovir cream, 5%. Int. J. Pharm. 2018, 535, 217–227. [Google Scholar] [CrossRef]
- Miranda, M.; Sousa, J.; Veiga, F.; Cardoso, C.; Vitorino, C. Bioequivalence of topical generic products. Part 1: Where are we now? Eur. J. Pharm. Sci. 2018, 123, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Sousa, J.; Veiga, F.; Cardoso, C.; Vitorino, C. Bioequivalence of topical generic products. Part 2. Paving the way to a tailored regulatory system. Eur. J. Pharm. Sci. 2018, 122, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Katragadda, U. Common Deficiencies-OGD Considerations. In Proceedings of the FDA workshop Complex Generic Drug Product Development Workshop, Maryland, MD, USA, 25 September 2019. [Google Scholar]
- Kikwai, L.; Tran, D.; Hauck, W.W.; Shah, V.P.; Stippler, E.S. Effect of Various Operational Parameters on Drug Release from a 1% Hydrocortisone Semisolid Dosage Form Using the Vertical Diffusion Cell Apparatus. Dissolution Technol. 2012, 19, 6–13. [Google Scholar] [CrossRef]
- Naik, P.; Shah, S.; Heaney, J.; Hanson, R.; Nagarsenker, M. Influence of Test Parameters on Release Rate of Hydrocortisone from Cream: Study Using Vertical Diffusion Cell. Dissolution Technol. 2016, 23, 14–20. [Google Scholar] [CrossRef]
- CDER. Validation of Chromatographic Methods; CDER: Washington, DC, USA, 1994. [Google Scholar]
- ICH. ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Int. Conf. Harmon. 2005, 1994, 17. [Google Scholar]
- Kregar, M.L.; Dürrigl, M.; Rožman, A.; Jelcic, Z.; Cetina-Čižmek, B.; Filipović-Grčić, J. Development and validation of an in vitro release method for topical particulate delivery systems. Int. J. Pharm. 2015, 485, 202–214. [Google Scholar] [CrossRef]
- USP Topical and Transdermal Drug Products—Product Performance Tests. Pharmacopeial Forum 2009, 35, 12–28.
- Basso, J.; Mendes, M.; Cova, T.; Sousa, J.; Pais, A.A.C.C.; Vitorino, C. Analytical Quality by Design (AQbD) as a multiaddressable platform for co-encapsulating drug assays. Anal. Methods 2018, 10, 5659–5671. [Google Scholar] [CrossRef]
- Vitorino, C.; Alves, L.; Antunes, F.; Sousa, J.; Pais, A.A.C.C. Design of a dual nanostructured lipid carrier formulation based on physicochemical, rheological, and mechanical properties. J. Nanoparticle Res. 2013, 15, 15. [Google Scholar] [CrossRef]
- Baert, B.; Boonen, J.; Burvenich, C.; Roche, N.; Stillaert, F.; Blondeel, P.; Van Boxclaer, J.; De Spiegeleer, B. A new discriminative criterion for the development of Franz diffusion tests for transdermal pharmaceuticals. J. Pharm. Pharm. Sci. 2010, 13, 218–230. [Google Scholar] [CrossRef]
- Faria, M.J.; Machado, R.; Ribeiro, A.; Gonçalves, H.; Oliveira, M.E.C.R.; Viseu, T.M.; Das Neves, J.; Lúcio, M. Rational Development of Liposomal Hydrogels: A Strategy for Topical Vaginal Antiretroviral Drug Delivery in the Context of HIV Prevention. Pharmaceutics 2019, 11, 485. [Google Scholar] [CrossRef] [Green Version]
- Krishnaiah, Y.S.R.; Xu, X.; Rahman, Z.; Yang, Y.; Katragadda, U.; Lionberger, R.; Peters, J.R.; Uhl, K.; Khan, M. Development of performance matrix for generic product equivalence of acyclovir topical creams. Int. J. Pharm. 2014, 475, 110–122. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry: Nonsterile Semisolid Dosage Forms: Scale-up and Postapproval Changes: Chemistry, Manufacturing, and Controls: In Vitro Release Testing and In Vivo Bioequivalence Documentation; FDA: Washington, DC, USA, 1997. [Google Scholar]
- Papadoyannis, I.N.; Samanidou, V.F. Validation of HPLC Instrumentation. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 753–783. [Google Scholar] [CrossRef]
- Vitorino, C.; Sousa, J.; Pais, A.A.C.C. A rapid reversed-phase HPLC method for the simultaneous analysis of olanzapine and simvastatin in dual nanostructured lipid carriers. Anal. Methods 2013, 5, 5058–5064. [Google Scholar] [CrossRef]
- Gonçalves, J.; Alves, G.; Bicker, J.; Falcão, A.; Fortuna, A. Development and full validation of an innovative HPLC-diode array detection technique to simultaneously quantify lacosamide, levetiracetam and zonisamide in human plasma. Bioanalysis 2018, 10, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, O.; Blanco, M.E.; Iriarte, G.; Bartolomé, L.; Maguregui, M.I.; Alonso, R.M.; Alonso, R.M. Bioanalytical chromatographic method validation according to current regulations, with a special focus on the non-well defined parameters limit of quantification, robustness and matrix effect. J. Chromatogr. A 2014, 1353, 10–27. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2(R1); ICH: Geneva, Switzerland, 2005. [Google Scholar]
- USP Validation of compendial methods. In United States Pharmacopeia and National Formulary; U.S. Pharmacopeia: Rockville, MD, USA, 2005; pp. 2748–2751. ISBN 1-889788-25-2.
- Carrer, V.; Guzmán, B.; Marti, M.; Alonso, C.; Coderch, L. Lanolin-Based Synthetic Membranes as Percutaneous Absorption Models for Transdermal Drug Delivery. Pharmaceutics 2018, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Council of Europe. Shutdown of European Pharmacopoeia, 9th ed.; Council of Europe: Strasburg, France, 2019.
- De Melo, E.K.S.; De Araujo, T.P.; Da Silva, J.W.V.; Chagas, S.; Bedor, D.C.G.; De Santana, D.P.; Leal, L.B. Metronidazole thermogel improves retention and decreases permeation through the skin. Braz. J. Pharm. Sci. 2017, 53, 1–9. [Google Scholar]
- Braddy, A.C.; Davit, B.M.; Stier, E.M.; Conner, D.P. Survey of International Regulatory Bioequivalence Recommendations for Approval of Generic Topical Dermatological Drug Products. AAPS J. 2014, 17, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Chang, R.-K.; Raw, A.; Lionberger, R.; Yu, L. Generic Development of Topical Dermatologic Products, Part II: Quality by Design for Topical Semisolid Products. AAPS J. 2013, 15, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.; Cardoso, C.; Vitorino, C. Quality and Equivalence of Topical Products: A Critical Appraisal. Eur. J. Pharm. Sci. 2019, 105082. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]



| Bifonazole | Column: XBridgeTM C18 5 µm (2.1 × 150 mm) |
| Mobile phase: Buffer solution (2 mL of phosphoric acid with 980 mL of ultrapurified water, adjusted to pH 3.2 ± 0.05 with trimethylamine) and acetonitrile (68:32, v/v) | |
| Flow: 0.5 mL/min | |
| Run time: 5.5 | |
| Wavelength: 210 nm | |
| Clotrimazole | Column: XBridgeTM C18 5 µm (2.1 × 150 mm) |
| Mobile phase: Methanol: 25 mM dipotassium hydrogen phosphate 75:25 m v/v, pH = 7.5 | |
| Flow: 0.4 mL/min | |
| Run time: 5.5 min | |
| Wavelength: 210 nm | |
| Tioconazole | Column: XBridgeTM C18 5 µm (2.1 × 150 mm) |
| Mobile phase: water: methanol (20:80, v/v) | |
| Flow: 0.4 mL/min | |
| Run time: 5.5 min | |
| Wavelength: 218 nm | |
| Etofenamate | Column: LiChrospher® 100 RP-18. 5 µm (4.6 mm × 125 mm) |
| Mobile phase: methanol: acetic acid 2% (80:20, v/v) | |
| Flow: 1 mL/min | |
| Run time: 5.5 min | |
| Wavelength: 287 nm | |
| Sodium diclofenac | Column: LiChrospher® 100 RP-18. 5 µm (4.6 mm × 125 mm) |
| Mobile phase: methanol: 2% acetic acid (75:25, v/v) | |
| Flow: 1 mL/min | |
| Run time: 7 min | |
| Wavelength: 280 nm | |
| Clobetasol proprionate | Column: LiChrospher® 100 RP-18. 5 µm (4.6 mm × 125 mm) |
| Mobile phase: methanol: 2% acetic acid (70:30, v/v) | |
| Flow: 0.35 mL/min | |
| Run time: 5.5 min | |
| Wavelength: 240 nm | |
| Hydrocortisone | Column: XBridgeTM C18 5 µm (2.1 × 150 mm) |
| Mobile phase: water and acetonitrile (75:25, v/v) | |
| Flow: 0.35 mL/min | |
| Run time: 8 min | |
| Wavelength: 247 nm | |
| Acyclovir | Column: XBridgeTM C18 5 µm (2.1 × 150 mm) |
| Mobile phase: water and methanol (95:5, v/v) | |
| Flow: 0.4 mL/min | |
| Run time: 6 min | |
| Wavelength: 247 nm |
| Bifonazole | PBS/ethanol (50:50, v/v, pH = 7.4) |
| Clotrimazole | PBS/ethanol (50:50, v/v, pH = 7.4) |
| Tioconazole | PBS/ethanol (50:50, v/v, pH = 4.5) |
| Etofenamate | PBS/ethanol (70:30, v/v) |
| Diclofenac | PBS/ethanol (80:20, v/v, pH = 7.4) |
| Clobetasol | PBS/ethanol (50:50, v/v, pH = 7.4) |
| Hydrocortisone | Water/ethanol (70:30, v/v) |
| Acyclovir | PBS |
| Drug | Calibration Curve Standards (µg/mL) | Quality Control Standards (µg/mL) | LOQ and LOD Standards (µg/mL) |
|---|---|---|---|
| Bifonazole | 0.1, 0.5, 1, 2.5, 5, 10, 25, 50, 75, 100, and 150 | 0.25, 20, and 80 | The calibration curve standards were used |
| Clotrimazole | 0.05, 0.25, 0.5, 1, 5, 10, 25, 50, 75, and 100 | 0.1, 20, and 40 | 0.05, 0.25, 0.5,1, and 5 |
| Tioconazole | 0.1, 0.5, 1, 2.5, 10, 25, and 50 | 4, 8, and 16 | The calibration curve standards were used |
| Etofenamate | 10, 25, 50, 75, 100, and 200 | 20, 80, and 180 | 0.5, 1, 2, 2.5, 4, and 5 |
| Diclofenac | 10, 25, 75, 100, 150, and 200 | 20, 80, and 180 | 0.5, 1, 2, 2.5, 4, and 5 |
| Clobetasol | 0.1, 0.25, 0.5, 1, 2, 5, and 10 | 0.1, 1.5, and 4 | The calibration curve standards were used |
| Hydrocortisone | 2.5, 5, 10, 20, 25, and 50 | 3, 15, and 40 | 0.25, 0.5, 1, 2, and 2.5 |
| Acyclovir | 5, 10, 25, 50, 75, 100, 150, and 300 | 20, 80, and 200 | 0.25, 0.5, 1, 2, and 5 |
| Standards | IVRT Samples | |
|---|---|---|
| Retention Time Mean (min) | ||
| BFZ | 3.947 ± 0.09 | 4.012 ± 0.06 |
| CLT | 4.315 ± 0.04 | 4.296 ± 0.02 |
| TCZ | 3.697 ± 0.11 | 4.064 ± 0.21 |
| ETF | 3.395 ± 0.02 | 3.390 ± 0.01 |
| DF | 4.166 ± 0.02 | 4.241 ± 0.01 |
| CLB | 4.241 ± 0.03 | 4.229 ± 0.04 |
| HC | 6.387 ± 0.02 | 6.438 ± 0.01 |
| ACV | 3.432 ± 0.07 | 3.506 ± 0.03 |
| Drug | Conc. | Retention Time (min) | Peak Area | T. Plates | K′ | T. Factor | Resolution | ||
|---|---|---|---|---|---|---|---|---|---|
| (µg/mL) | Mean | RSD | Mean | RSD | |||||
| BFZ | 50 | 3.973 | 0.09 | 9,135,580 | 0.08 | 1,898,400 | 3.057 | 1.684 | 2.09 |
| CLT | 50 | 4.262 | 0.07 | 8,701,986 | 0.13 | 1,721,502 | 2.393 | 1.634 | 4.10 |
| TCZ | 10 | 3.643 | 0.08 | 522,268 | 0.44 | 1,289,836 | 2.060 | 1.889 | 4.86 |
| ETF | 100 | 3.491 | 0.24 | 2,306,243 | 0.14 | 1,883,362.8 | 1.957 | 1.140 | 5.70 |
| DF | 100 | 4.202 | 0.05 | 2,188,617 | 0.42 | 1,598,293 | 2.628 | 1.177 | 5.67 |
| CLB | 2 | 4.230 | 0.19 | 96,246 | 1.12 | 1,368,004 | 1.812 | 1.703 | 7.50 |
| HC | 25 | 6.378 | 0.06 | 1,779,896 | 0.05 | 2,689,997 | 4.446 | 1.456 | 15.77 |
| ACV | 100 | 3.350 | 0.23 | 8,187,120 | 0.57 | 1,362,134 | 1.795 | 1.583 | 3.60 |
| Acceptance criteria | - | ≤2.0% | - | ≤2.0% | >1000 | >1000 | ≤2.0 | >2.0 | |
| Drugs | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|
| BFZ | 1.46 | 4.43 |
| CLT | 0.35 | 1.06 |
| TCZ | 14.05 | 42.59 |
| ETF | 0.44 | 1.33 |
| DF | 0.88 | 0.29 |
| CLB | 2.52 | 7.63 |
| HC | 0.05 | 0.15 |
| ACV | 0.04 | 0.12 |
| Drug | Mean R2 | Mean Slope | Mean Intercept |
|---|---|---|---|
| BFZ | 0.9984 ± 0.0007 | 71,972,704 ± 846,636 | 12,227,244 ± 6,063,944 |
| CLT | 0.9994 ± 0.0002 | 175,242 ± 5754 | 1659 ± 749 |
| TCZ | 0.9987 ± 0.0003 | 61,944 ± 2704 | 3271 ± 231 |
| ETF | 0.9988 ± 0.0008 | 23,376 ± 1055 | 56,591 ± 31,185 |
| DF | 0.9994 ± 0.0003 | 21,958 ± 106 | 10,595 ± 14,365 |
| CLB | 0.9970 ± 0.0008 | 52,833 ± 1014 | 1160 ± 429 |
| HC | 0.9999 ± 0.0001 | 72,492 ± 772 | −2643 ± 834 |
| ACV | 0.9996 ± 0.0001 | 73,690 ± 2119 | 10,926 ± 8039 |
| Intraday (n = 5) | Interday (n = 15) | ||||||
|---|---|---|---|---|---|---|---|
| Drug | Concentration CQ (µg/mL) | Measured Concentration (µg/mL) | Accuracy (%) | RSD (%) | Measured Concentration (µg/mL) | Accuracy (%) | RSD (%) |
| BFZ | 0.25 | 0.26 ± 0.02 | −5.41 | 7.97 | 0.26 ± 0.03 | −4.92 | 11.52 |
| 20 | 20.2 ± 0.4 | −1.22 | 1.93 | 20.2 ± 0.5 | −1.23 | 2.72 | |
| 80 | 82.5 ± 1.5 | −3.13 | 1.77 | 82.6 ± 2.9 | −3.33 | 3.52 | |
| CLT | 0.1 | 0.11 ± 0.01 | −3.25 | 12.28 | 0.11 ± 0.01 | −3.25 | 12.91 |
| 20 | 19.4 ± 0.4 | 9.07 | 1.86 | 19.5 ± 0.8 | 9.07 | 4.08 | |
| 40 | 38.9 ± 1.2 | 3.03 | 2.97 | 38.6 ± 2.8 | 3.56 | 7.37 | |
| TCZ | 4 | 3.82 ± 0.13 | 4.51 | 3.42 | 3.8 ± 0.29 | 5.06 | 7.76 |
| 8 | 7.68 ± 0.14 | 3.96 | 1.94 | 7.67 ± 0.30 | 4.19 | 3.94 | |
| 16 | 16.4 ± 0.4 | −2.30 | 2.42 | 16.4 ± 0.6 | −2.30 | 3.76 | |
| ETF | 20 | 20.9 ± 0.9 | −0.05 | 4.62 | 20.6 ± 2.1 | −0.03 | 10.27 |
| 80 | 79.2 ± 1.9 | 0.01 | 2.47 | 79.2 ± 2.6 | 0.01 | 3.49 | |
| 180 | 175 ± 3 | 0.03 | 2.11 | 175 ± 7 | −1.19 | 4.08 | |
| DF | 20 | 20.1 ± 0.3 | −0.01 | 1.52 | 20.1 ± 0.5 | −0.01 | 2.52 |
| 80 | 83.31 ± 0.99 | −0.04 | 1.17 | 83.3 ± 0.5 | −0.04 | 3.95 | |
| 180 | 181 ± 3 | −0.01 | 1.92 | 181 ± 4 | −1.26 | 2.41 | |
| CLB | 0.4 | 0.38 ± 0.02 | 3.75 | 7.22 | 0.39 ± 0.05 | 3.36 | 13.04 |
| 1.5 | 1.38 ± 0.09 | 7.69 | 7.01 | 1.38 ± 0.15 | 8.30 | 10.71 | |
| 4 | 3.75 ± 0.16 | 6.21 | 4.39 | 3.75 ± 0.27 | 6.31 | 7.11 | |
| HC | 3 | 3.18 ± 0.04 | −6.11 | 1.23 | 3.16 ± 0.17 | −5.23 | 5.53 |
| 15 | 15.11 ± 0.2 | −0.72 | 1.48 | 15.1 ± 0.3 | −0.67 | 2.04 | |
| 40 | 40.2 ± 0.2 | −0.61 | 0.54 | 40.2 ± 0.5 | −0.55 | 1.18 | |
| ACV | 20 | 20.7 ± 0.9 | −3.48 | 4.47 | 20.6 ± 1.3 | −2.84 | 6.27 |
| 80 | 80.2 ± 0.9 | −0.28 | 1.19 | 80.2 ± 1.4 | −0.22 | 1.80 | |
| 200 | 203 ± 1 | −1.72 | 0.56 | 204 ± 7 | −1.87 | 3.61 | |
| Drug | Concentration CQ (µg/mL) | Mean Concentration (µg/mL) | Accuracy (%) | RSD (%) |
|---|---|---|---|---|
| BFZ | 0.25 | 0.26 ± 0.02 | 6.29 | 9.15 |
| 20 | 18.4 ± 0.8 | −8.05 | 4.44 | |
| 80 | 81.2 ± 0.5 | 1.49 | 0.59 | |
| CLT | 0.1 | 0.11 ± 0.01 | 9.03 | 9.85 |
| 20 | 17.5 ± 0.7 | −12.46 | 3.92 | |
| 40 | 35.6 ± 0.3 | −10.87 | 0.72 | |
| TCZ | 4 | 3.99 ± 0.07 | 0.13 | 1.71 |
| 8 | 7.56 ± 0.28 | 5.47 | 3.76 | |
| 16 | 16.0 ± 0.7 | −0.08 | 4.12 | |
| ETF | 20 | 22.9 ± 0.5 | 13.77 | 2.37 |
| 80 | 79 ± 1.4 | −0.93 | 1.75 | |
| 180 | 171 ± 0 | −4.95 | 0.24 | |
| DF | 20 | 21.0 ± 0.3 | 4.84 | 1.36 |
| 80 | 85.3 ± 1.0 | 6.62 | 1.21 | |
| 180 | 197 ± 10 | 9.18 | 5.05 | |
| CLB | 0.4 | 0.41 ± 0.05 | 3.59 | 11.75 |
| 1.5 | 1.37 ± 0.04 | −8.75 | 3.09 | |
| 4 | 3.61 ± 0.04 | −9.83 | 1.17 | |
| HC | 3 | 3.44 ± 0.06 | 14.67 | 1.86 |
| 15 | 15.2 ± 0.3 | 2.18 | 2.16 | |
| 40 | 40.1 ± 0.2 | 0.70 | 0.17 | |
| ACV | 5 | 4.59 ± 0.02 | −8.17 | 0.63 |
| 100 | 95.9 ± 0.1 | −4.02 | 0.08 | |
| 200 | 206 ± 0 | 2.94 | 0.12 |
| AUTOSAMPLER | SHORT TERM STABILITY | ||||||
|---|---|---|---|---|---|---|---|
| Drugs | Concentration CQ (µg/mL) | Mean Concentration (µg/mL) | Accuracy (%) | RSD (%) | Mean Concentration (µg/mL) | Accuracy (%) | RSD (%) |
| BFZ | 0.25 | 0.23 ± 0.01 | −8.43 | 6.29 | 0.29 ± 0.01 | 15.96 | 3.94 |
| 20 | 19.9 ± 0.3 | −0.29 | 1.64 | 20.7 ± 0.4 | 3.17 | 2.07 | |
| 80 | 84.2 ± 0.8 | 5.22 | 0.94 | 85.5 ± 0.8 | 6.83 | 0.96 | |
| CLT | 0.1 | 0.11 ± 0.01 | 7.67 | 5.37 | 0.10 ± 0.01 | −0.41 | 13.45 |
| 20 | 19.5 ± 0.2 | −2.68 | 0.94 | 20.2 ± 0.8 | 1.14 | 4.08 | |
| 40 | 38.7 ± 0.6 | −3.30 | 1.44 | 40.5 ± 3.3 | 1.26 | 8.10 | |
| TCZ | 4 | 3.41 ± 0.15 | −14.81 | 4.50 | 3.49 ± 0.18 | −12.73 | 5.19 |
| 8 | 7.76 ± 0.63 | −2.94 | 8.12 | 7.41 ± 0.35 | −7.33 | 4.75 | |
| 16 | 16.3 ± 0.3 | 1.72 | 1.57 | 17.4 ± 1.2 | 8.98 | 6.72 | |
| ETF | 20 | 21.9 ± 0.7 | −0.09 | 3.06 | 20.2 ± 0.1 | 1.03 | 0.75 |
| 80 | 92.8 ± 3.0 | −0.16 | 3.28 | 78.8 ± 1.7 | −1.54 | 2.20 | |
| 180 | 198 ± 5 | −0.10 | 2.66 | 178 ± 1 | −0.87 | 0.35 | |
| DF | 20 | 20.4 ± 0.6 | 2.01 | 3.00 | 20.0 ± 0.1 | 0.04 | 0.67 |
| 80 | 84.4 ± 2.7 | 5.50 | 3.20 | 82.3 ± 0.6 | 2.82 | 0.69 | |
| 180 | 180 ± 5 | −0.15 | 2.60 | 184 ± 0.0 | 2.05 | 0.20 | |
| CLB | 0.4 | 0.34 ± 0.01 | −14.13 | 2.70 | 0.33 ± 0.02 | −16.24 | 6.19 |
| 1.5 | 1.35 ± 0.03 | −10.01 | 2.02 | 1.41 ± 0.06 | −5.94 | 3.99 | |
| 4 | 3.72 ± 0.08 | −7.06 | 2.18 | 3.91 ± 0.21 | −2.24 | 5.43 | |
| HC | 3 | 3.06 ± 0.05 | 1.96 | 1.77 | 3.40 ± 0.05 | 13.36 | 1.66 |
| 15 | 15.1 ± 0.5 | 0.82 | 3.25 | 15.2 ± 0.3 | 1.24 | 2.04 | |
| 40 | 39.9 ± 0.3 | −0.31 | 0.79 | 40.1 ± 0.7 | 0.21 | 0.27 | |
| ACV | 20 | 21.8 ± 1.7 | 8.80 | 7.83 | 22.7 ± 0.3 | 13.50 | 1.42 |
| 80 | 87.6 ± 0.5 | 9.55 | 0.57 | 85.3 ± 0.8 | 6.59 | 0.93 | |
| 200 | 215 ± 0.0 | 7.78 | 0.14 | 209 ± 2 | 4.27 | 0.77 | |
| API | MW | Log P | S (mg/mL) | pKa | H donors | H Acceptors | Lipinski Rule | Log D | Solubility and pH (mg/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.6 | 6.5 | 7.4 | 4.6 | 6.5 | 7.4 | ||||||||
| CLT | 344.84 | 5.84 | 0.000703 | 6.26 | 0 | 1 | X | 5.3 | 5.7 | 5.8 | 0.03 | 0 | 0 |
| BFZ | 310.40 | 5.23 | 0.000791 | 6.36 | 0 | 1 | X | 4.7 | 5.07 | 5.2 | 0.05 | 0 | 0 |
| TCZ | 387.70 | 5.30 | 0.000552 | 6.48 | 0 | 2 | X | 4.72 | 5.1 | 5.3 | 4.6 | 0 | 0 |
| ETF | 369.34 | 4.86 | 0.0187 | 15.12 | 2 | 4 | C | 4.9 | 4.9 | 4.9 | 0.02 | 0.02 | 0.02 |
| DF | 296.15 | 4.26 | 0.0149 | 4.00 | 2 | 3 | C | 3.6 | 1.8 | 1.1 | 0.07 | 4.8 | 37.9 |
| CLB | 466.97 | 4.18 | 0.0017 | 13.59 | 1 | 4 | C | 4.2 | 4.2 | 4.2 | 0 | 0 | 0 |
| HC | 362.47 | 1.28 | 0.408 | 12.59 | 3 | 5 | C | 1.3 | 1.3 | 1.3 | 0.4 | 0.4 | 0.4 |
| ACV | 225.21 | −1.03 | 9.1 | 11.98 | 3 | 7 | C | −1.04 | −1.03 | −1.03 | 9.3 | 9.1 | 9.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, M.; Cardoso, C.; Vitorino, C. Fast Screening Methods for the Analysis of Topical Drug Products. Processes 2020, 8, 397. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8040397
Miranda M, Cardoso C, Vitorino C. Fast Screening Methods for the Analysis of Topical Drug Products. Processes. 2020; 8(4):397. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8040397
Chicago/Turabian StyleMiranda, Margarida, Catarina Cardoso, and Carla Vitorino. 2020. "Fast Screening Methods for the Analysis of Topical Drug Products" Processes 8, no. 4: 397. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8040397