Preparation of Spray-Dried Functional Food: Effect of Adding Bacillus clausii Bacteria as a Co-Microencapsulating Agent on the Conservation of Resveratrol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
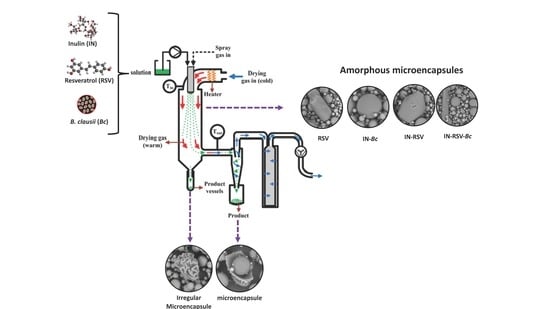
2.2. Preparation of Spray-Dried Functional Foods
2.3. Culturability of Bacillus clausii in the Microencapsulated Functional Food
2.4. Radical Scavenging Activity of the Functional Food
2.5. Scanning Electron Microscopy (SEM) of the Microencapsulated Powders
2.6. Statistical Analysis
3. Results and Discussion
3.1. Culturability of Spray Dried Functional Foods
3.2. Radical Scavenging Activity
3.3. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.T.; Li, X.; Xie, M.L.; Huang, Z.; Huang, Y.X.; Wu, G.X.; Peng, Z.R.; Sun, Y.N.; Ming, Q.L.; Liu, Y.X.; et al. Resveratrol: Review on its discovery, anti-leukemia effects and pharmacokinetics. Chem. Biol. Interact. 2019, 306, 29–38. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Pormohammad, A.; Chirani, A.S.; Pouriran, R.; Erfanimanesh, S.; Hashemi, A. Comprehensive review on the antimicrobial potency of the plant polyphenol Resveratrol. Biomed. Pharmacother. 2017, 95, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Gøbel, R.; Larsen, N.; Mølgaard, C.; Jakobsen, M.; Michaelsen, K.F. Probiotics to young children with atopic dermatitis: A randomized placebo-controlled trial. Int. J. Probiotics Prebiotics 2010, 5, 53–59. [Google Scholar]
- Guo, Q.; Goldenberg, J.Z.; Humphrey, C.; El Dib, R.; Johnston, B.C. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Effect of encapsulation methods on the physicochemical properties and the stability of Lactobacillus plantarum (NCIM 2083) in synbiotic powders and in-vitro digestion conditions. J. Food Eng. 2020, 283, 110033. [Google Scholar] [CrossRef]
- Althans, D.; Schrader, P.; Enders, S. Solubilisation of quercetin: Comparison of hyperbranched polymer and hydrogel. J. Mol. Liq. 2014, 196, 86–93. [Google Scholar] [CrossRef]
- Velasco, J.; Dobarganes, M.C.; Márquez-Ruiz, G. Antioxidant activity of phenolic compounds in sunflower oil-in-water emulsions containing sodium caseinate and lactose. Eur. J. Lipid Sci. Technol. 2004, 106, 325–333. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Carlan, I.; Blaga, A.; Rocha, F. Soluble vitamins (vitamin B12 and vitamin C) microencapsulated with different biopolymers by a spray drying process. Powder Technol. 2016, 289, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.; Chawla, P.; Arora, S.; Tomar, S.K.; Singh, A.K. Iron microencapsulation with blend of gum arabic, maltodextrin and modified starch using modified solvent evaporation method-Milk fortification. Food Hydrocoll. 2015, 43, 622–628. [Google Scholar] [CrossRef]
- Ton, M.N.; Tran, T.T.; Le, V. Microencapsulation of rambutan seed oil by spray-drying using different protein preparations. Int. Food Res. J. 2016, 23, 123–128. [Google Scholar]
- Calinoiu, L.F.; Ştefanescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan coating applications in probiotic microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Araujo-Díaz, S.B.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, Z. Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carbohydr. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.D.; Fernández, M.A.; Díaz, D.D. Recent Strategies in Resveratrol Delivery Systems. Chempluschem 2019, 84, 951–973. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fan, Q.; Liu, T.; Wusigale; Liang, L. Co-encapsulation of α-tocopherol and resveratrol in oil-in-water emulsion stabilized by sodium caseinate: Impact of polysaccharide on the stability and bioaccessibility. J. Food Eng. 2020, 264. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Gu, G.; Guo, Z. Extraction, degree of polymerization determination and prebiotic effect evaluation of inulin from Jerusalem artichoke. Carbohydr. Polym. 2015, 121, 315–319. [Google Scholar] [CrossRef]
- Caleffi, E.R.; Krausová, G.; Hyršlová, I.; Paredes, L.L.R.; dos Santos, M.M.; Sassaki, G.L.; Gonçalves, R.A.C.; de Oliveira, A.J.B. Isolation and prebiotic activity of inulin-type fructan extracted from Pfaffia glomerata (Spreng) Pedersen roots. Int. J. Biol. Macromol. 2015, 80, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Apolinário, A.C.; de Carvalho, E.M.; de Lima Damasceno, B.P.G.; da Silva, P.C.D.; Converti, A.; Pessoa, A.; da Silva, J.A. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crops Prod. 2017, 108, 355–362. [Google Scholar] [CrossRef]
- Fox, P.F. Lactose: Chemistry and properties. In Advanced Dairy Chemistry; Springer: New York, NY, USA, 2009; Volume 3, pp. 1–15. ISBN 9780387848648. [Google Scholar]
- Sessa, M.; Tsao, R.; Liu, R.; Ferrari, G.; Donsì, F. Evaluation of the Stability and Antioxidant Activity of Nanoencapsulated Resveratrol during in Vitro Digestion. J. Agric. Food Chem. 2011, 59, 12352–12360. [Google Scholar] [CrossRef]
- Koga, C.C.; Andrade, J.E.; Ferruzzi, M.G.; Lee, Y. Stability of Trans -Resveratrol Encapsulated in a Protein Matrix Produced Using Spray Drying to UV Light Stress and Simulated Gastro-Intestinal Digestion. J. Food Sci. 2016, 81, C292–C300. [Google Scholar] [CrossRef] [PubMed]
- Peñalva, R.; Morales, J.; González-Navarro, C.; Larrañeta, E.; Quincoces, G.; Peñuelas, I.; Irache, J. Increased Oral Bioavailability of Resveratrol by Its Encapsulation in Casein Nanoparticles. Int. J. Mol. Sci. 2018, 19, 2816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, M.; Rodríguez-Rojo, S.; Alves-Santos, F.M.; Cocero, M.J. Encapsulation of resveratrol on lecithin and β-glucans to enhance its action against Botrytis cinerea. J. Food Eng. 2015, 165, 13–21. [Google Scholar] [CrossRef]
- Trotta, V.; Lee, W.-H.; Loo, C.-Y.; Haghi, M.; Young, P.M.; Scalia, S.; Traini, D. In vitro biological activity of resveratrol using a novel inhalable resveratrol spray-dried formulation. Int. J. Pharm. 2015, 491, 190–197. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.Z.; Leyva-Porras, C.; Martínez-Guerra, E.; Pérez-García, S.A.; Aguilar-Martínez, J.A.; Álvarez-Salas, C. Physical properties of inulin and inulin-orange juice: Physical characterization and technological application. Carbohydr. Polym. 2014, 105, 10–19. [Google Scholar] [CrossRef]
- Toneli, J.; Park, K.; Negreiros, A.; Murr, F. Spray-drying process optimization of chicory root inulin. Dry. Technol. 2010, 28, 369–379. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Saavedra-Leos, M.Z.; Cervantes-González, E.; Aguirre-Bañuelos, P.; Silva-Cázarez, M.B.; Álvarez-Salas, C. Spray Drying of Blueberry Juice-Maltodextrin Mixtures: Evaluation of Processing Conditions on Content of Resveratrol. Antioxidants 2019, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Rochín-Medina, J.J.; Ramírez-Medina, H.K.; Rangel-Peraza, J.G.; Pineda-Hidalgo, K.V.; Iribe-Arellano, P. Use of whey as a culture medium for Bacillus clausii for the production of protein hydrolysates with antimicrobial and antioxidant activity. Food Sci. Technol. Int. 2018, 24, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Zhu, H.; Zhang, Z.; Yang, S.; Li, H. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J. Cereal Sci. 2015, 64, 92–99. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation Efficiency of Food Flavours and Oils during Spray Drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Reineccius, G.A. Spray-Drying of Food Flavors. In Flavor Encapsulation; American Chemical Society: Washington, DC, USA, 1988; pp. 55–66. ISBN 9780841214828. [Google Scholar]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Howes, T. Problems Associated With Spray Drying Of Sugar-Rich Foods. Dry. Technol. 1997, 15, 671–684. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J. Spray drying of nopal mucilage (Opuntia ficus-indica): Effects on powder properties and characterization. Carbohydr. Polym. 2010, 81, 864–870. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Tantratian, S.; Pradeamchai, M. Select a protective agent for encapsulation of Lactobacillus plantarum. LWT 2020, 123, 109075. [Google Scholar] [CrossRef]
- Hayrapetyan, H.; Abee, T.; Nierop Groot, M. Sporulation dynamics and spore heat resistance in wet and dry biofilms of Bacillus cereus. Food Control 2016, 60, 493–499. [Google Scholar] [CrossRef]
- De Araujo-Uribe, N.; Ruiz-Villadiego, O.S.; Montoya-Campuzano, O.I.; Ramírez-Gutiérrez, L.A. Viability of probiotic bacteria Bacillus Polymyxa, Bacillus Megaterium and Lactobacillus Delbruekii subsp. bulgaricus microencapsulated under the spray-drying technique. DYNA 2018, 85, 272–276. [Google Scholar] [CrossRef]
- Utami, D.A.; Suprayudi, M.A. Quality of Dried Bacillus NP5 and Its Effect on Growth Performance of Tilapia (Oreochromis niloticus). Pakistan J. Biol. Sci. 2015, 18, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Romano, N.; Mobili, P.; Zuñiga-Hansen, M.E.; Gómez-Zavaglia, A. Physico-chemical and structural properties of crystalline inulin explain the stability of Lactobacillus plantarum during spray-drying and storage. Food Res. Int. 2018, 113, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Raddatz, G.C.; Poletto, G.; de Deus, C.; Codevilla, C.F.; Cichoski, A.J.; Jacob-Lopes, E.; Muller, E.I.; Flores, E.M.M.; Esmerino, E.A.; de Menezes, C.R. Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Res. Int. 2020, 130, 108902. [Google Scholar] [CrossRef]
- Maleki, O.; Khaledabad, M.A.; Amiri, S.; Asl, A.K.; Makouie, S. Microencapsulation of Lactobacillus rhamnosus ATCC 7469 in whey protein isolate-crystalline nanocellulose-inulin composite enhanced gastrointestinal survivability. LWT 2020, 126, 109224. [Google Scholar] [CrossRef]
- And, C.I.; Kailasapathy, K. Effect of Co-encapsulation of Probiotics with Prebiotics on Increasing the Viability of Encapsulated Bacteria under In Vitro Acidic and Bile Salt Conditions and in Yogurt. J. Food Sci. 2005, 70, M18–M23. [Google Scholar] [CrossRef]
- Corona-Hernandez, R.I.; Álvarez-Parrilla, E.; Lizardi-Mendoza, J.; Islas-Rubio, A.R.; de la Rosa, L.A.; Wall-Medrano, A. Structural Stability and Viability of Microencapsulated Probiotic Bacteria: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 614–628. [Google Scholar] [CrossRef]
- Crittenden, R.; Weerakkody, R.; Sanguansri, L.; Augustin, M. Synbiotic Microcapsules That Enhance Microbial Viability during Nonrefrigerated Storage and Gastrointestinal Transit. Appl. Environ. Microbiol. 2006, 72, 2280–2282. [Google Scholar] [CrossRef] [Green Version]
- Xavier dos Santos, D.; Casazza, A.A.; Aliakbarian, B.; Bedani, R.; Saad, S.M.I.; Perego, P. Improved probiotic survival to in vitro gastrointestinal stress in a mousse containing Lactobacillus acidophilus La-5 microencapsulated with inulin by spray drying. LWT 2019, 99, 404–410. [Google Scholar] [CrossRef]
- Li, R.; Roos, Y.H.; Miao, S. Characterization of mechanical and encapsulation properties of lactose/maltodextrin/WPI matrix. Food Hydrocoll. 2017, 63, 149–159. [Google Scholar] [CrossRef]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef] [Green Version]
- Silanikove, N.; Leitner, G.; Merin, U. The Interrelationships between Lactose Intolerance and the Modern Dairy Industry: Global Perspectives in Evolutional and Historical Backgrounds. Nutrients 2015, 7, 7312–7331. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.-H.; Li, X.-R. Microencapsulation properties of soy protein isolate: Influence of preheating and/or blending with lactose. J. Food Eng. 2013, 117, 281–290. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Bhan, N.; Masuko, S.; James, P.; Wood, J.; McCallum, S.; Linhardt, R.J.; Dordick, J.S.; Koffas, M.A.G. Antimicrobial mechanism of resveratrol- trans -dihydrodimer produced from peroxidase-catalyzed oxidation of resveratrol. Biotechnol. Bioeng. 2015, 112, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.L.; Tan, L.T.-H.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Chuah, L.-H.; Ming, L.C.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Resveratrol—Potential Antibacterial Agent against Foodborne Pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhaes Junior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, V.; Altomare, A.; Guarino, M.P.L.; Locato, V.; Cocca, S.; Cimini, S.; Palma, R.; Alloni, R.; De Gara, L.; Cicala, M. Antioxidant Activity of Inulin and Its Role in the Prevention of Human Colonic Muscle Cell Impairment Induced by Lipopolysaccharide Mucosal Exposure. PLoS ONE 2014, 9, e98031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, H.-M.; Zhou, H.-Z.; Yang, J.-Y.; Li, R.; Song, H.; Wu, H.-X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef] [Green Version]
- Phisut, N.; Jiraporn, B. Characteristics and antioxidant activity of Maillard reaction products derived from chitosan-sugar solution. Int. Food Res. J. 2013, 20, 1077–1085. [Google Scholar]
- Silva, E.K.; Zabot, G.L.; Bargas, M.A.; Meireles, M.A.A. Microencapsulation of lipophilic bioactive compounds using prebiotic carbohydrates: Effect of the degree of inulin polymerization. Carbohydr. Polym. 2016, 152, 775–783. [Google Scholar] [CrossRef]
- Ha, H.-K.; Jeon, N.-E.; Kim, J.W.; Han, K.-S.; Yun, S.S.; Lee, M.-R.; Lee, W.-J. Physicochemical Characterization and Potential Prebiotic Effect of Whey Protein Isolate/Inulin Nano Complex. Korean J. Food Sci. Anim. Resour. 2016, 36, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Sonam, K.S.; Guleria, S. Synergistic Antioxidant Activity of Natural Products. Ann. Pharmacol. Pharm. 2017, 2, 1–6. [Google Scholar]
- Rochín-Medina, J.J.; Ramírez, K.; Rangel-Peraza, J.G.; Bustos-Terrones, Y.A. Increase of content and bioactivity of total phenolic compounds from spent coffee grounds through solid state fermentation by Bacillus clausii. J. Food Sci. Technol. 2018, 55, 915–923. [Google Scholar] [CrossRef]
- Kotowicz, N.; Bhardwaj, R.K.; Ferreira, W.T.; Hong, H.A.; Olender, A.; Ramirez, J.; Cutting, S.M. Safety and probiotic evaluation of two Bacillus strains producing antioxidant compounds. Benef. Microbes 2019, 10, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Fajardo-Cavazos, P.; Rebeil, R.; Slieman, T.A.; Riesenman, P.J.; Law, J.F.; Xue, Y. Bacterial endospores and their significance in stress resistance. Anton. Leeuw. Int. J. G. 2002, 81, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Riesenman, P.J.; Nicholson, W.L. Role of the Spore Coat Layers in Bacillus subtilis Spore Resistance to Hydrogen Peroxide, Artificial UV-C, UV-B, and Solar UV Radiation. Appl. Environ. Microbiol. 2000, 66, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Isticato, R.; Ricca, E.; Baccigalupi, L. Spore Adsorption as a Nonrecombinant Display System for Enzymes and Antigens. J. Vis. Exp. 2019, e59102. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Tachikawa, H.; Gao, X.-D.; Nakanishi, H. Use of Yeast Spores for Microencapsulation of Enzymes. Appl. Environ. Microbiol. 2014, 80, 4502–4510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spizzirri, U.G.; Altimari, I.; Puoci, F.; Parisi, O.I.; Iemma, F.; Picci, N. Innovative antioxidant thermo-responsive hydrogels by radical grafting of catechin on inulin chain. Carbohydr. Polym. 2011, 84, 517–523. [Google Scholar] [CrossRef]
- Littringer, E.M.; Mescher, A.; Schröttner, H.; Walzel, P.; Urbanetz, N.A.; Ii, I. Tailoring particle morphology of spray dried mannitol carrier particles by variation of the outlet temperature. In Proceedings of the 23rd Annual Conference on Liquid Atomization and Spray Systems (Ilas-Europe), Brno, Czech Republic, 6–9 September 2010; Volume 94, pp. 1–5. [Google Scholar]
- Tobin, J.T.; Fitzsimons, S.M.; Kelly, A.L.; Kelly, P.M.; Auty, M.A.E.; Fenelon, M.A. Microparticulation of mixtures of whey protein and inulin. Int. J. Dairy Technol. 2010, 63, 32–40. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Maldonado, D.; Espinosa-Solis, V.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Martinez-Gutierrez, F.; Román-Aguirre, M.; Saavedra-Leos, M.Z. Preparation of Spray-Dried Functional Food: Effect of Adding Bacillus clausii Bacteria as a Co-Microencapsulating Agent on the Conservation of Resveratrol. Processes 2020, 8, 849. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8070849
Vázquez-Maldonado D, Espinosa-Solis V, Leyva-Porras C, Aguirre-Bañuelos P, Martinez-Gutierrez F, Román-Aguirre M, Saavedra-Leos MZ. Preparation of Spray-Dried Functional Food: Effect of Adding Bacillus clausii Bacteria as a Co-Microencapsulating Agent on the Conservation of Resveratrol. Processes. 2020; 8(7):849. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8070849
Chicago/Turabian StyleVázquez-Maldonado, Daniel, Vicente Espinosa-Solis, César Leyva-Porras, Patricia Aguirre-Bañuelos, Fidel Martinez-Gutierrez, Manuel Román-Aguirre, and María Z. Saavedra-Leos. 2020. "Preparation of Spray-Dried Functional Food: Effect of Adding Bacillus clausii Bacteria as a Co-Microencapsulating Agent on the Conservation of Resveratrol" Processes 8, no. 7: 849. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8070849