Antioxidant Molecules from Plant Waste: Extraction Techniques and Biological Properties
Abstract
:1. Introduction
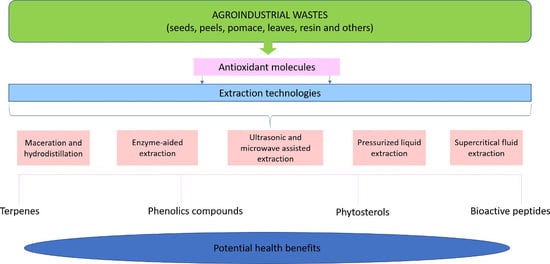
2. Plant Waste as Source of Bioactive Compounds
3. Bioactive Compounds from Plant Waste
3.1. Terpenes
3.2. Phenolic Compounds
3.3. Phytosterols
3.4. Bioactive Peptides
4. Extraction Techniques for Recovery of Bioactive Compounds
4.1. Maceration and Hydrodistillation
4.2. Enzyme-Aided Extraction
4.3. Ultrasonic and Microwave Assisted Extraction
4.4. Pressurized Liquid Extraction
4.5. Supercritical Fluid Extraction
5. Potential Health Benefits of Recovered Bioactive Compounds of Plant Waste
5.1. Antioxidant Properties
5.2. Anti-Obesity and Anti-Diabetic
5.3. Anti-Hypertensive
5.4. Anti-Cancer
5.5. Anti-Bacterial
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Strategic Work of FAO for Sustainable Food and Agriculture. Available online: http://www.fao.org/3/a-i6488e.pdf (accessed on 12 November 2020).
- FAO. The State of Food and Agriculture Moving Forward on Food Loss and Waste Reduction. Available online: http://www.fao.org/3/ca6030en/ca6030en.pdf (accessed on 12 November 2020).
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. Australas. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- FAO. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value Added Products. Available online: http://www.fao.org/3/i3273e/i3273e.pdf (accessed on 12 November 2020).
- Belc, N.; Mustatea, G.; Apostol, L.; Iorga, S.; Vlăduţ, V.-N.; Mosoiu, C. Cereal supply chain waste in the context of circular economy. In Proceedings of the 8th International Conference on Thermal Equipment, Renewable Energy and Rural Development (TE-RE-RD 2019), Târgovişte, Romania, 20 August 2019; p. 8. [Google Scholar]
- Leyva-López, N.; Lizárraga-Velázquez, C.E.; Hernández, C.; Sánchez-Gutiérrez, E.Y. Exploitation of agro-industrial waste as potential source of bioactive compounds for aquaculture. Foods 2020, 9, 843. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- González-López, Á.M.; Quiñones-Aguilar, E.E.; Rincón-Enríquez, G. Actividad biológica de los terpenos en el área agroalimentaria. In Los Compuestos Bioactivos y Tecnologías de Extracción; Espinosa Andrews, H., García Marquez, E., Gastelum Martínez, E., Eds.; Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A. C. (CIATEJ): Guadalajara, Mexico, 2016. [Google Scholar]
- Görgüç, A.; Özer, P.; Yılmaz, F.M. Microwave-assisted enzymatic extraction of plant protein with antioxidant compounds from the food waste sesame bran: Comparative optimization study and identification of metabolomics using LC/Q-TOF/MS. J. Food Process. Preserv. 2020, 44, e14304. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Alongi, M.; Melchior, S.; Anese, M. Reducing the glycemic index of short dough biscuits by using apple pomace as a functional ingredient. LWT Food Sci. Technol. 2019, 100, 300–305. [Google Scholar] [CrossRef]
- Dang, Y.; Zhou, T.; Hao, L.; Cao, J.; Sun, Y.; Pan, D. In vitro and in vivo studies on the angiotensin-converting enzyme inhibitory activity peptides isolated from broccoli protein hydrolysate. J. Agric. Food Chem. 2019, 67, 6757–6764. [Google Scholar] [CrossRef]
- Guesmi, F.; Prasad, S.; Tyagi, A.K.; Landoulsi, A. Antinflammatory and anticancer effects of terpenes from oily fractions of Teucruim alopecurus, blocker of IκBα kinase, through downregulation of NF-κB activation, potentiation of apoptosis and suppression of NF-κB-regulated gene expression. Biomed. Pharmacother. 2017, 95, 1876–1885. [Google Scholar] [CrossRef]
- Gautam, M.; Thapa, R.K.; Gupta, B.; Soe, Z.C.; Ou, W.; Poudel, K.; Jin, S.G.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Phytosterol-loaded CD44 receptor-targeted PEGylated nano-hybrid phyto-liposomes for synergistic chemotherapy. Expert Opin. Drug Del. 2020, 17, 423–434. [Google Scholar] [CrossRef]
- Santos, I.B.; de Bem, G.F.; Cordeiro, V.S.C.; da Costa, C.A.; de Carvalho, L.; da Rocha, A.P.M.; da Costa, G.F.; Ognibene, D.T.; de Moura, R.S. Supplementation with Vitis vinifera L. skin extract improves insulin resistance and prevents hepatic lipid accumulation and steatosis in high-fat diet-fed mice. Nutr. Res. 2017, 43, 69–81. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of natural antioxidants from agro-industrial side streams through advanced extraction techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. In vitro bioaccessibility and functional properties of polyphenols from pomegranate peels and pomegranate peels-enriched cookies. Food Funct. 2016, 7, 4247–4258. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. 2019, 25, 6631–6638. [Google Scholar] [CrossRef]
- Gajendran, B.; Durai, P.; Varier, K.M.; Chinnasamy, A. A novel phytosterol isolated from Datura inoxia, RinoxiaB is a potential cure colon cancer agent by targeting BAX/Bcl2 pathway. Bioorg. Med. Chem. 2020, 28, 115242. [Google Scholar] [CrossRef]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzua, C.; Perez, D.; Dicenta, S.; de la Cerda, P.M.; Amigo, L.; Carreno, J.C.; Echeverria, G.; et al. The consumption of beef burgers prepared with wine grape pomace flour improves fasting glucose, plasma antioxidant levels, and oxidative damage markers in humans: A controlled trial. Nutrients 2018, 10, 1388. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits by-products-A source of valuable active principles. A short review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- González-García, E.; Puchalska, P.; Marina, M.L.; García, M.C. Fractionation and identification of antioxidant and angiotensin-converting enzyme-inhibitory peptides obtained from plum (Prunus domestica L.) stones. J. Funct. Food. 2015, 19, 376–384. [Google Scholar] [CrossRef]
- He, R.; Yang, Y.-J.; Wang, Z.; Xing, C.-r.; Yuan, J.; Wang, L.-F.; Udenigwe, C.; Ju, X.-R. Rapeseed protein-derived peptides, LY, RALP, and GHS, modulates key enzymes and intermediate products of renin–angiotensin system pathway in spontaneously hypertensive rat. NPJ Sci. Food 2019, 3, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.-J.; Gasmalla, M.A.A.; Li, P.; Yang, R. Enzyme-assisted extraction processing from oilseeds: Principle, processing and application. Innov. Food Sci. Emerg. Technol. 2016, 35, 184–193. [Google Scholar] [CrossRef]
- Moayedi, A.; Mora, L.; Aristoy, M.C.; Safari, M.; Hashemi, M.; Toldrá, F. Peptidomic analysis of antioxidant and ACE-inhibitory peptides obtained from tomato waste proteins fermented using Bacillus Subtilis. Food Chem. 2018, 250, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Vaásquez-Villanueva, R.; Orellana, J.M.A.; Marina, M.L.; García, M.C. Isolation and characterization of angiotensin converting enzyme inhibitory peptides from peach seed hydrolysates: In vivo assessment of antihypertensive activity. J. Agric. Food Chem. 2019, 67, 10313–10320. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Li, G. ACE-inhibitory and antioxidant peptides from coconut cake albumin hydrolysates: Purification, identification and synthesis. RSC Adv. 2019, 9, 5925–5936. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.F.; Zhou, J.X.; Liu, X.J.; Zhang, Y.B.; Cai, S.B. Digestive enzyme inhibition of different phenolic fractions and main phenolic compounds of ultra-high-pressure-treated palm fruits: Interaction and molecular docking analyses. J. Food Qual. 2020, 2020, 8811597. [Google Scholar] [CrossRef]
- Teixeira, S.D.; Fiorio, J.L.; Galvan, D.; Sefstrom, C.; Cogo, P.M.; Sales Junior, V.; Rodrigues, M.B.; Hendges, A.P.P.K.; Maia, B.H.L.d.N.S. Investigation on chemical composition and optimization of essential oil obtainment from waste Pinus taeda L. using hydrodistillation. Braz. Arch. Biol. Technol. 2016, 59, e16150043. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.C.; Jorge, N. Bioactive compounds of oils extracted from fruits seeds obtained from agroindustrial waste. Eur. J. Lipid Sci. Technol. 2017, 119, 1600024. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, T. Phytosterols in cereal by-products. J. Am. Oil Chem. Soc. 2005, 82, 439–444. [Google Scholar] [CrossRef]
- Uddin, M.; Ferdosh, S.; Haque Akanda, M.J.; Ghafoor, K.; Rukshana, A.; Ali, M.E.; Kamaruzzaman, B.; Fauzi, M.; Shaarani, S.; Islam Sarker, M.Z. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar] [CrossRef]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recent advances in the recovery techniques of plant-based proteins from agro-industrial by-products. Food Rev. Int. 2020, 1–22. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech. 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matus, M.F.; Jorquera-Román, M.; Zúñiga-Hernández, J. Anti-proliferative effect of terpenes on human prostate cancer cells: Natural sources and their potential role as chemopreventive agents. Rev. Chil. Nutr. 2017, 44, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of terpenoids: Monoterpenes, sesquiterpenes and diterpenes. In Annual Plant Reviews Online, 2nd ed.; Wink, M., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2010; Volume 40, pp. 258–303. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281520, Humulene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Humulene (accessed on 19 November 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6654, Alpha-Pinene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Pinene (accessed on 20 November 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 16061204, Lutein. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lutein-G (accessed on 19 November 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 446925, Lycopene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Lycopene (accessed on 19 November 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280863, Kaempferol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Kaempferol (accessed on 9 September 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 370, Gallic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Gallic-acid (accessed on 19 November 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 73160, Catechin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Catechin (accessed on 20 November 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 72276, Epicatechin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Epicatechin (accessed on 19 November 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 173183, Campesterol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Campesterol (accessed on 19 November 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 222284, beta-Sitosterol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/beta-Sitosterol (accessed on 19 November 2020).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280794, Stigmasterol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Stigmasterol (accessed on 19 November 2020).
- Schrader, J.; Bohlmann, J. Biotechnology of Isoprenoids; Springer: Cham, Switzerland, 2015; Volume 149. [Google Scholar]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Contreras-Angulo, L.A.; Emus-Medina, A.; Heredia, J.B. Terpenes in oregano: Constituents, extraction, analysis and biological properties. In Terpenes: Biosynthesis, Applications and Research; Bjarke, A., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2018; pp. 1–64. [Google Scholar]
- Barreto, R.S.; Quintans, J.S.; Amarante, R.K.; Nascimento, T.S.; Amarante, R.S.; Barreto, A.S.; Pereira, E.W.; Duarte, M.C.; Coutinho, H.D.; Menezes, I.R. Evidence for the involvement of TNF-α and IL-1β in the antinociceptive and anti-inflammatory activity of Stachys lavandulifolia Vahl. (Lamiaceae) essential oil and (-)-α-bisabolol, its main compound, in mice. J. Ethnopharmacol. 2016, 191, 9–18. [Google Scholar] [CrossRef]
- Sarmento-Neto, J.F.; Do Nascimento, L.G.; Felipe, C.F.B.; De Sousa, D.P. Analgesic potential of essential oils. Molecules 2016, 21, 20. [Google Scholar] [CrossRef]
- Gouveia, D.N.; Pina, L.T.; Rabelo, T.K.; da Rocha Santos, W.B.; Quintans, J.S.S.; Guimaraes, A.G. Monoterpenes as perspective to chronic pain management: A systematic review. Curr. Drug Targets 2018, 19, 960–972. [Google Scholar] [CrossRef]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef]
- Dutra, R.C.; da Silva, K.A.B.S.; Bento, A.F.; Marcon, R.; Paszcuk, A.F.; Meotti, F.C.; Pianowski, L.F.; Calixto, J.B. Euphol, a tetracyclic triterpene produces antinociceptive effects in inflammatory and neuropathic pain: The involvement of cannabinoid system. Neuropharmacology 2012, 63, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.G.; Araujo, A.A.; Quintans, J.S.; Sluka, K.A.; Quintans-Junior, L.J. Enhanced analgesic activity by cyclodextrins–a systematic review and meta-analysis. Expert Opin. Drug Del. 2015, 12, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Sánchez, D.O.; Zamilpa, A.; Pérez, S.; Herrera-Ruiz, M.; Tortoriello, J.; González-Cortazar, M.; Jiménez-Ferrer, E. Effect of hautriwaic acid isolated from Dodonaea viscosa in a model of kaolin/carrageenan-induced monoarthritis. Planta Med. 2015, 81, 1240–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, T.; Xu, W.; Liang, L.; Li, J.; Ding, X.; Chen, X.; Hu, J.; Lv, A.; Li, X. Clinical efficacy of andrographolide sulfonate in the treatment of severe hand, foot, and mouth disease (HFMD) is dependent upon inhibition of neutrophil activation. Phytother. Res. 2015, 29, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.S.; Nørgaard, A.W.; Koponen, I.K.; Sørli, J.B.; Paidi, M.D.; Hansen, S.W.; Clausen, P.A.; Nielsen, G.D.; Wolkoff, P.; Larsen, S.T. Limonene and its ozone-initiated reaction products attenuate allergic lung inflammation in mice. J. Immunotoxicol. 2016, 13, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Li, D.; Zhou, D.; Wang, D.; Liu, Q.; Fan, S. Chemical composition, antibacterial activity and related mechanism of the essential oil from the leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016, 194, 698–705. [Google Scholar] [CrossRef]
- Montironi, I.D.; Cariddi, L.N.; Reinoso, E.B. Evaluation of the antimicrobial efficacy of Minthostachys verticillata essential oil and limonene against Streptococcus uberis strains isolated from bovine mastitis. Rev. Arg. Microbiol. 2016, 48, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Zhang, L.; Cao, G.; Feng, J.; Yue, M.; Xu, Y.; Dai, B.; Han, Q.; Guo, X. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 2020, 97, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Carotenoid research: History and new perspectives for chemistry in biological systems. BBA-Mol. Cell. Biol. L. 2020, 1865, 158699. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Zhang, H.; Lim, L.-S.; Ma, H.; Li, S.; Zheng, H. Roles of carotenoids in invertebrate immunology. Front. Immunol. 2020, 10. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of hydrophilic and lipophilic antioxidants from mango peel (Mangifera indica L. cv. Ataulfo) on lipid peroxidation in fish oil. CyTA-J. Food 2018, 16, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Bajpai, V.K.; Shukla, S. Sesquiterpenes and cytotoxicity. In Natural products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3515–3550. [Google Scholar]
- Langhasova, L.; Hanusova, V.; Rezek, J.; Stohanslova, B.; Ambroz, M.; Kralova, V.; Vanek, T.; Lou, J.D.; Yun, Z.L.; Yang, J.; et al. Essential oil from Myrica rubra leaves inhibits cancer cell proliferation and induces apoptosis in several human intestinal lines. Ind. Crop. Prod. 2014, 59, 20–26. [Google Scholar] [CrossRef]
- Vermerris, W.; Nicholson, R. Families of phenolic compounds and means of classification. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006; pp. 1–34. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Review: Dietary phenolic compounds, health benefits and bioaccessibility. Archivos Latinoamericanos de Nutrición 2016, 66, 87–100. [Google Scholar]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef]
- Gylling, H.; Simonen, P. Phytosterols, phytostanols, and lipoprotein metabolism. Nutrients 2015, 7, 7965–7977. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, N.; Khan, W.; Shadab, M.; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.A.; Abdel-Wahab, A.F. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Miras-Moreno, B.; Sabater-Jara, A.B.; Pedreño, M.; Almagro, L. Bioactivity of phytosterols and their production in plant in vitro cultures. J. Agric. Food Chem. 2016, 64, 7049–7058. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, G.P. Phytosterols, lipid administration, and liver disease during parenteral nutrition. JPEN J. Parenter. Enteral. Nutr. 2015, 39, 39S–60S. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocolloid. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Lin, Y.; Knol, D.; Trautwein, E.A. Phytosterol oxidation products (POP) in foods with added phytosterols and estimation of their daily intake: A literature review. Eur. J. Lipid Sci. Technol. 2016, 118, 1423–1438. [Google Scholar] [CrossRef] [Green Version]
- Ogbe, R.J.; Ochalefu, D.O.; Mafulul, S.G.; Olaniru, O.B. A review on dietary phytosterols: Their occurrence, metabolism and health benefits. Asian J. Plant Sci. Res. 2015, 5, 10–21. [Google Scholar]
- Ramprasath, V.R.; Awad, A.B. Role of phytosterols in cancer prevention and treatment. J. AOAC Int. 2015, 98, 735–738. [Google Scholar] [CrossRef]
- Plat, J.; Hendrikx, T.; Bieghs, V.; Jeurissen, M.L.; Walenbergh, S.M.; van Gorp, P.J.; De Smet, E.; Konings, M.; Vreugdenhil, A.C.; Guichot, Y.D. Protective role of plant sterol and stanol esters in liver inflammation: Insights from mice and humans. PLoS ONE 2014, 9, e110758. [Google Scholar] [CrossRef]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Res. Int. 2020, 132, 109086. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Meneguetti, B.T.; Machado, L.D.S.; Oshiro, K.G.N.; Nogueira, M.L.; Carvalho, C.M.E.; Franco, O.L. Antimicrobial peptides from fruits and their potential use as biotechnological tools—A review and outlook. Front. Microbiol. 2017, 7, 2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva Dantas, C.C.; de Souza, E.L.; Cardoso, J.D.; de Lima, L.A.; de Sousa Oliveira, K.; Migliolo, L.; Dias, S.C.; Franco, O.L.; Magnani, M. Identification of a napin-like peptide from Eugenia malaccensis L. Seeds with inhibitory activity toward Staphylococcus aureus and Salmonella enteritidis. Protein J. 2014, 33, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M. Chapter 11-Terpenoids A2-Badal, Simone. In Pharmacognosy; Academic Press: Boston, MA, USA, 2017; pp. 233–266. [Google Scholar]
- Yadav, N.; Yadav, R.; Goyal, A. Chemistry of terpenoids. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 272–278. [Google Scholar]
- Uysal, S.; Cvetanović, A.; Zengin, G.; Zeković, Z.; Mahomoodally, M.F.; Bera, O. Optimization of maceration conditions for improving the extraction of phenolic compounds and antioxidant effects of Momordica charantia L. leaves through response surface methodology (RSM) and artificial neural networks (ANNs). Anal. Lett. 2019, 52, 2150–2163. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.D.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA-J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Gololo, S.S.; Shai, L.J.; Sethoga, L.; Agyei, N.; Bassey, K.E.; Mogale, M.A. Isolation of a mixture of phytosterol compounds from the n-Hexane extract of Jatropha lagarinthoides (Sond) collected from Zebediela sub-region in Limpopo province, South Africa. J. Chem. Pharm. Sci. 2016, 9, 3084–3087. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 extraction and antioxidant activity of lycopene and β-carotene-enriched oleoresin from tomato (Lycopersicum esculentum L.) peels by-product of a Tunisian industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Anastácio, A.; Silva, R.; Carvalho, I.S. Phenolics extraction from sweet potato peels: Modelling and optimization by response surface modelling and artificial neural network. J. Food Sci. Tech. 2016, 53, 4117–4125. [Google Scholar] [CrossRef] [PubMed]
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use natural deep eutectic solvents as efficient green reagents to extract procyanidins and anthocyanins from cranberry pomace and predictive modeling by RSM and artificial neural networking. Sep. Purifi. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Wu, F.; Jin, Y.; Xu, X.; Yang, N. Electrofluidic pretreatment for enhancing essential oil extraction from citrus fruit peel waste. J. Clean. Prod. 2017, 159, 85–94. [Google Scholar] [CrossRef]
- Bustamante, J.; van Stempvoort, S.; García-Gallarreta, M.; Houghton, J.A.; Briers, H.K.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Clean. Prod. 2016, 137, 598–605. [Google Scholar] [CrossRef]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Tech. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purifi. Technol. 2019, 219, 55–63. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L.; Stupić, D.; Preiner, D.; Ašperger, D.; Kontić, J.K. Recovery of flavonoids from grape skins by enzyme-assisted extraction. Sep. Sci. Technol. 2016, 51, 255–268. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; González-Delgado, Á.D.; García-Martinez, J.B.; L’Abbate, P. Optimization of enzyme-assisted extraction of flavonoids from corn husks. Processes 2019, 7, 804. [Google Scholar] [CrossRef] [Green Version]
- Leyva-López, N.; Valdez-Torres, B.; Delgado-Vargas, F.; García-Estrada, R.S.; Heredia, J.B. Optimized enzyme-aided extraction enhances recovery of carotenoids from tomato peel and improves the biological activity. Int. J. Pharma. Bio. Sci. 2017, 8, 721–727. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- Esteve, C.; Marina, M.; García, M. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015, 167, 272–280. [Google Scholar] [CrossRef]
- Fuglsang, A.; Rattray, F.P.; Nilsson, D.; Nyborg, N.C. Lactic acid bacteria: Inhibition of angiotensin converting enzyme in vitro and in vivo. Antonie Van Leeuwenhoek. 2003, 83, 27–34. [Google Scholar] [CrossRef]
- Karami, Z.; Peighambardoust, S.; Hesari, J.; Akbari-Adergani, B. Response surface methodology to optimize hydrolysis parameters in production of antioxidant peptides from wheat germ protein by alcalase digestion and identification of antioxidant peptides by LC-MS/MS. J. Agricultur. Sci. Technol. 2019, 21, 829–844. [Google Scholar]
- Sonawane, S.K.; Arya, S.S. Citrullus lanatus protein hydrolysate optimization for antioxidant potential. J. Food Meas. Charact. 2017, 11, 1834–1843. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Dubey, K.K.; Singhal, R.S. Improvements in the extraction of bioactive compounds by enzymes. Curr. Opin. Food Sci. 2019, 25, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef] [PubMed]
- González-García, E.; Marina, M.L.; García, M.C. Plum (Prunus domestica, L.) by-product as a new and cheap source of bioactive peptides: Extraction method and peptides characterization. J. Funct. Food. 2014, 11, 428–437. [Google Scholar] [CrossRef]
- Jahanbani, R.; Ghaffari, S.M.; Salami, M.; Vahdati, K.; Sepehri, H.; Sarvestani, N.N.; Sheibani, N.; Moosavi-Movahedi, A.A. Antioxidant and anticancer activities of walnut (Juglans regia L.) protein hydrolysates using different proteases. Plant. Food Hum. Nutr. 2016, 71, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Chiozzi, R.Z.; Laganà, A. Characterization of antioxidant and angiotensin-converting enzyme inhibitory peptides derived from cauliflower by-products by multidimensional liquid chromatography and bioinformatics. J. Funct. Food 2018, 44, 40–47. [Google Scholar] [CrossRef]
- Thamnarathip, P.; Jangchud, K.; Jangchud, A.; Nitisinprasert, S.; Tadakittisarn, S.; Vardhanabhuti, B. Extraction and characterisation of R iceberry bran protein hydrolysate using enzymatic hydrolysis. Intern. J. Food Sci. Techol. 2016, 51, 194–202. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Arya, S.S. Bioactive L. acidissima protein hydrolysates using Box–Behnken design. 3 Biotech 2017, 7, 218. [Google Scholar] [CrossRef]
- Yu, Q.; Fan, L.; Li, J. A novel process for asparagus polyphenols utilization by ultrasound assisted adsorption and desorption using resins. Ultrason. Sonochem. 2020, 63, 104920. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Tech. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, C.; Ma, Y.; Jia, M. Degradation behavior of polyphenols in model aqueous extraction system based on mechanical and sonochemical effects induced by ultrasound. Sep. Purifi. Technol. 2020, 247, 116967. [Google Scholar] [CrossRef]
- Grassino, A.N.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crop. Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Chmelová, D.; Škulcová, D.; Legerská, B.; Horník, M.; Ondrejovič, M. Ultrasonic-assisted extraction of polyphenols and antioxidants from Picea abies bark. J. Biotechnol. 2020, 314–315, 25–33. [Google Scholar] [CrossRef]
- Wang, L.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Ultrasound assisted purification of polyphenols of apple skins by adsorption/desorption procedure. Ultrason. Sonochem. 2019, 55, 18–24. [Google Scholar] [CrossRef]
- Carbone, K.; Amoriello, T.; Iadecola, R. Exploitation of kiwi juice pomace for the recovyer of natural antioxidants through microwave-assisted extraction. Agriculture 2020, 10, 435. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Ortega-Barbosa, J.P.; Sánchez-Camargo, A.D.P.; Rodríguez-Varela, L.I.; Parada-Alfonso, F. Pressurized Liquid Extraction of Bioactives. In Reference Module in Food Science; Elsevier: Berkeley, CA, USA, 2020; pp. 1–15. [Google Scholar]
- Wianowska, D.; Gil, M. Critical approach to PLE technique application in the analysis of secondary metabolites in plants. TrAC Trend. Anal. Chem. 2019, 114, 314–325. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Liu, X.; Yuan, F.; Gao, Y. Antioxidative phenolics obtained from spent coffee grounds (Coffea arabica L.) by subcritical water extraction. Ind. Crop. Prod. 2015, 76, 946–954. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Cao, Y.; Zheng, G. Optimization of subcritical water extraction of phenolic antioxidants from pomegranate (Punica granatum L.) peel by response surface methodology. Anal. Methods 2017, 9, 4647–4656. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Xu, H.; Xu, C.; Yuan, F.; Knez, Ž.; Novak, Z.; Gao, Y. Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC–ABTS+ assay. Food Bioprod. Process. 2012, 90, 215–223. [Google Scholar] [CrossRef]
- Saravana, P.S.; Getachew, A.T.; Ahmed, R.; Cho, Y.-J.; Lee, Y.-B.; Chun, B.-S. Optimization of phytochemicals production from the ginseng by-products using pressurized hot water: Experimental and dynamic modelling. Biochem. Eng. J. 2016, 113, 141–151. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. TrAC Trend. Anal. Chem. 2019, 116, 236–247. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Qi, F.; Zhou, J.; Cen, K. Subcritical water hydrolysis of rice straw for reducing sugar production with focus on degradation by-products and kinetic analysis. Bioresour. Technol. 2015, 186, 8–14. [Google Scholar] [CrossRef]
- Munir, M.T.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Plaza, M.; Mendiola, J.A.; Ibáñez, E.; Herrero, M. Chapter 4–Subcritical water extraction and neoformation of antioxidants. In Water Extraction of Bioactive Compounds; Dominguez González, H., González Muñoz, M.J., Eds.; Elsevier: Berkeley, CA, USA, 2017; pp. 109–130. [Google Scholar]
- Chen, Y.-T.; Kao, W.-T.; Lin, K.-W. Effects of pH on the total phenolic compound, antioxidative ability and the stability of dioscorin of various yam cultivars. Food Chem. 2008, 107, 250–257. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A. 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [Green Version]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trend. Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, F.W.F.; de Oliveira, M.S.; Bezerra, P.N.; Cunha, V.M.B.; Silva, M.P.; da Costa, W.A.; Pinto, R.H.H.; Cordeiro, R.M.; da Cruz, J.N.; Chaves Neto, A.M.J.; et al. Extraction of bioactive compounds. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, A.M., Isloor, A.M., Eds.; Elsevier: Berkeley, CA, USA, 2020; Chapter 8; pp. 149–167. [Google Scholar]
- Gallego, R.; Bueno, M.; Herrero, M. Sub-and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae–An update. TrAC Trend. Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Chun, B.S. Optimization of carotenoids and antioxidant activity of oils obtained from a co-extraction of citrus (Yuzu ichandrin) by-products using supercritical carbon dioxide. Biomass Bioenerg. 2017, 106, 1–7. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P.; Boumghar, Y. Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. LWT Food Sci. Technol. 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Kitrytė, V.; Bagdonaitė, D.; Rimantas Venskutonis, P. Biorefining of industrial hemp (Cannabis sativa L.) threshing residues into cannabinoid and antioxidant fractions by supercritical carbon dioxide, pressurized liquid and enzyme-assisted extractions. Food Chem. 2018, 267, 420–429. [Google Scholar] [CrossRef]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Bonikowski, R.; Balawejder, M. Optimization of extraction process of antioxidant compounds from yellow onion skin and their use in functional bread production. LWT 2020, 117, 108614. [Google Scholar] [CrossRef]
- Gil-Chávez, G.J.; Villa, J.A.; Ayala-Zavala, F.J.; Heredia, J.B.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food. Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’Donnell, C.P.; Martin-Diana, A.B.; Barry-Ryan, C. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason. Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Mandal, Y.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction–An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and analysis of polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Halliwell, B., Gutteridge, J.M.C., Eds.; Oxford University Press: Oxford, MS, USA, 2007; Volume 4, pp. 187–267. [Google Scholar]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillon, N.J.; Croze, M.L.; Vella, R.E.; Soulère, L.; Lagarde, M.; Soulage, C.O. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology 2012, 153, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Tremocoldi, M.A.; Rosalen, P.L.; Franchin, M.; Massarioli, A.P.; Denny, C.; Daiuto, É.R.; Paschoal, J.A.R.; Melo, P.S.; Alencar, S.M.D. Exploration of avocado by-products as natural sources of bioactive compounds. PLoS ONE 2018, 13, e0192577. [Google Scholar] [CrossRef] [Green Version]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of dietary intake of phenolic compounds from mango peel extract on growth, lipid peroxidation and antioxidant enzyme activities in zebrafish (Danio rerio). Lat. Am. J. Aquat. Res. 2019, 47, 602–611. [Google Scholar] [CrossRef] [Green Version]
- Reshmitha, T.R.; Thomas, S.; Geethanjali, S.; Arun, K.B.; Nisha, P. DNA and mitochondrial protective effect of lycopene rich tomato (Solanum lycopersicum L.) peel extract prepared by enzyme assisted extraction against H2O2 induced oxidative damage in L6 myoblasts. J. Funct. Food. 2017, 28, 147–156. [Google Scholar] [CrossRef]
- Álvarez, M.V.; Hincapié, S.; Saavedra, N.; Alzate, L.M.; Muñoz, A.M.; Cartagena, C.J.; Londoño-Londoño, J. Exploring feasible sources for lutein production: Food by-products and supercritical fluid extraction, a reasonable combination. Phytochem. Rev. 2015, 14, 891–897. [Google Scholar] [CrossRef]
- Chisté, R.C.; Freitas, M.; Mercadante, A.Z.; Fernandes, E. Carotenoids inhibit lipid peroxidation and hemoglobin oxidation, but not the depletion of glutathione induced by ROS in human erythrocytes. Life Sci. 2014, 99, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Azevedo, J.; Pereira, M.J.; Valentão, P.; Andrade, P.B. Chemical assessment and antioxidant capacity of pepper (Capsicum annuum L.) seeds. Food Chem. Toxicol. 2013, 53, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Alkhalaf, M.I.; Alansari, W.S.; Ibrahim, E.A.; Elhalwagy, M.E.A. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. Sci. 2019, 31, 1358–1362. [Google Scholar] [CrossRef]
- Vivancos, M.; Moreno, J.J. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic. Biol. Med. 2005, 39, 91–97. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Food. 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- García, M.C.; Endermann, J.; González-García, E.; Marina, M.L. HPLC-Q-TOF-MS identification of antioxidant and antihypertensive peptides recovered from cherry (Prunus cerasus L.) subproducts. J. Agric. Food Chem. 2015, 63, 1514–1520. [Google Scholar] [CrossRef]
- Rabe, K.; Lehrke, M.; Parhofer, K.G.; Broedl, U.C. Adipokines and insulin resistance. Mol. Med. 2008, 14, 741–751. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th Ed.; Brussels, Belgium; 2019. Available online: https://www.diabetesatlas.org/ (accessed on 19 November 2020).
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity telationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sanchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy-Basel 2017, 7, 47. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food. Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Balli, D.; Cecchi, L.; Khatib, M.; Bellumori, M.; Cairone, F.; Carradori, S.; Zengin, G.; Cesa, S.; Innocenti, M.; Mulinacci, N. Characterization of arils juice and peel decoction of fifteen varieties of Punica granatum L.: A focus on anthocyanins, ellagitannins and polysaccharides. Antioxidants 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef] [PubMed]
- El-Hadary, A.E.; Ramadan, M.F. Phenolic profiles, antihyperglycemic, antihyperlipidemic, and antioxidant properties of pomegranate (Punica granatum) peel extract. J. Food Biochem. 2019, 43, e12803. [Google Scholar] [CrossRef] [PubMed]
- Goss, M.J.; Nunes, M.L.O.; Machado, I.D.; Merlin, L.; Macedo, N.B.; Silva, A.M.O.; Bresolin, T.M.B.; Santin, J.R. Peel flour of Passiflora edulis Var. Flavicarpa supplementation prevents the insulin resistance and hepatic steatosis induced by low-fructose-diet in young rats. Biomed. Pharmacother. 2018, 102, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Lucci, P.; Nunez, O.; Tundis, R.; Balzano, M.; Frega, N.G.; Conte, L.; Moret, S.; Filatova, D.; Moyano, E.; et al. Native colombian fruits and their by-products: Phenolic profile, antioxidant activity and hypoglycaemic potential. Foods 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit (Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Liu, X.Y.; Liu, Z.Y.; Wu, S.F.; Shahidi, F.; Zhou, D.Y.; Zhu, B.W. Improvement of phenolic contents and antioxidant activities of longan (Dimocarpus longan) peel extracts by enzymatic treatment. Waste Biomass Valoriz. 2020, 11, 3987–4002. [Google Scholar] [CrossRef]
- Carullo, G.; Spizzirri, U.G.; Loizzo, M.R.; Leporini, M.; Sicari, V.; Aiello, F.; Restuccia, D. Valorization of red grape (Vitis vinifera cv. Sangiovese) pomase as functional food ingredient. Ital. J. Food Sci. 2020, 32, 367–385. [Google Scholar] [CrossRef]
- Ferri, M.; Bin, S.; Vallini, V.; Fava, F.; Michelini, E.; Roda, A.; Minnucci, G.; Bucchi, G.; Tassoni, A. Recovery of polyphenols from red grape pomace and assessment of their antioxidant and anti-cholesterol activities. New Biotech. 2016, 33, 338–344. [Google Scholar] [CrossRef]
- Kadouh, H.C.; Sun, S.; Zhu, W.J.; Zhou, K.Q. alpha-Glucosidase inhibiting activity and bioactive compounds of six red wine grape pomace extracts. J. Funct. Food. 2016, 26, 577–584. [Google Scholar] [CrossRef]
- Kilic, I.H.; Sarikurkcu, C.; Karagoz, I.D.; Uren, M.C.; Kocak, M.S.; Cilkiz, M.; Tepe, B. A significant by-product of the industrial processing of pistachios: Shell skin–RP-HPLC analysis, and antioxidant and enzyme inhibitory activities of the methanol extracts of Pistacia vera L. Shell skins cultivated in Gaziantep, Turkey. RSC Adv. 2016, 6, 1203–1209. [Google Scholar] [CrossRef]
- Lavelli, V.; Harsha, P.; Ferranti, P.; Scarafoni, A.; Iametti, S. Grape skin phenolics as inhibitors of mammalian alpha-glucosidase and alpha-amylase-effect of food matrix and processing on efficacy. Food Funct. 2016, 7, 1655–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Tu, Z.-C.; Xie, X.; Wang, H.; Wang, H.; Wang, Z.-X.; Sha, X.-M.; Lu, Y. Jackfruit (Artocarpus heterophyllus Lam.) peel: A better source of antioxidants and a-glucosidase inhibitors than pulp, flake and seed, and phytochemical profile by HPLC-QTOF-MS/MS. Food Chem. 2017, 234, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Haque, A.R.; Kabir, M.R.; Hasan, M.M.; Khushe, K.J.; Hasan, S.M.K. Fruit by-products: The potential natural sources of antioxidants and alpha-glucosidase inhibitors. J. Food Sci. Technol.-Mysore 2020, 1–12. [Google Scholar] [CrossRef]
- Henriquez, C.; Sarkar, D.; Molina, J.; Sepulveda, S.; Cordova, A.; Saavedra, J.; Shetty, K. Improving antioxidant and anti-hyperglycemic activity in cereal and apple-based food formulations using bioactive ingredients from apple peel. J. Food Process Preserv. 2020, 44, 1–11. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Akinyemi, A.J.; Henle, T.; Saliu, J.A.; Schwarzenbolz, U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J. Funct. Food. 2012, 4, 450–458. [Google Scholar] [CrossRef]
- Lim, S.M.; Loh, S.P. In vitro antioxidant capacities and antidiabetic properties of phenolic extracts from selected citrus peels. Int. Food Res. J. 2016, 23, 211–219. [Google Scholar]
- Ling, Y.; Shi, Z.; Yang, X.L.; Cai, Z.W.; Wang, L.X.; Wu, X.M.; Ye, A.Q.; Jiang, J.P. Hypolipidemic effect of pure total flavonoids from peel of Citrus (PTFC) on hamsters of hyperlipidemia and its potential mechanism. Exp. Gerontol. 2020, 130, 110786. [Google Scholar] [CrossRef]
- Toomer, O.T.; Vu, T.; Pereira, M.; Williams, K. Dietary supplementation with peanut skin polyphenolic extracts (PSPE) reduces hepatic lipid and glycogen stores in mice fed an atherogenic diet. J. Funct. Food. 2019, 55, 362–370. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Ghosh, A.K.; Ghosh, C. Recent developments on polyphenol–protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food Funct. 2012, 3, 592–605. [Google Scholar] [CrossRef] [PubMed]
- de la Garza, A.L.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velderrain-Rodriguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sanchez, M.; Astiazaran-Garcia, H.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Reactive species in disease: Friends or foes? In Free Radicals in Biology and Medicine; Halliwell, B., Gutteridge, J.M.C., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 511–638. [Google Scholar]
- Zhang, T.; Li, M.; Fu, X.; Mou, H. Purification and charicterization of angiotensin I-converting enzyme (ACE) inhibitory peptides with specific structure X-Pro. Eur. Food Res. Technol. 2019, 245, 1743–1753. [Google Scholar] [CrossRef]
- Turner, J.M.; Kodali, R. Should angiotensin-converting enzyme inhibitors ever be used for the management of hypertension? Curr. Cardiol. Rep. 2020, 22, 95. [Google Scholar] [CrossRef]
- Liu, R.L.; Ge, X.L.; Gao, X.Y.; Zhan, H.Y.; Shi, T.; Su, N.; Zhang, Z.Q. Two angiotensin-converting enzyme-inhibitory peptides from almond protein and the protective action on vascular endothelial function. Food Funct. 2016, 7, 3733–3739. [Google Scholar] [CrossRef]
- Gu, X.; Hou, Y.-K.; Li, D.; Wang, J.-Z.; Wang, F.-J. Separation, purification, and identification of angiotensin I–converting enzyme inhibitory peptides from walnut (Juglans regia L.) hydrolyzate. Int. J. Food Prop. 2015, 18, 266–276. [Google Scholar] [CrossRef]
- He, R.; Malomo, S.A.; Alashi, A.; Girgih, A.T.; Ju, X.; Aluko, R.E. Purification and hypotensive activity of rapeseed protein-derived renin and angiotensin converting enzyme inhibitory peptides. J. Funct. Food. 2013, 5, 781–789. [Google Scholar] [CrossRef]
- He, R.; Malomo, S.A.; Girgih, A.T.; Ju, X.; Aluko, R.E. Glycinyl-histidinyl-serine (GHS), a novel rapeseed protein-derived peptide has blood pressure-lowering effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2013, 61, 8396–8402. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, T.; Han, W.; Chen, W.; Zheng, X.; Wang, J. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from cauliflower by-products protein hydrolysate. Process Biochem. 2016, 51, 1299–1305. [Google Scholar] [CrossRef]
- Montone, C.M.; Zenezini Chiozzi, R.; Marchetti, N.; Cerrato, A.; Antonelli, M.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Laganà, A. Peptidomic approach for the identification of peptides with potential antioxidant and anti-hyperthensive effects derived from asparagus by-products. Molecules 2019, 24, 3627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vásquez-Villanueva, R.; Muñoz-Moreno, L.; Carmena, M.J.; Marina, M.L.; García, M.C. In vitro antitumor and hypotensive activity of peptides from olive seeds. J. Funct. Food. 2018, 42, 177–184. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, M.; Wang, Z.; Aluko, R.E.; He, R. Antihypertensive and antioxidant activities of enzymatic wheat bran protein hydrolysates. J. Food Biochem. 2020, 44, e13090. [Google Scholar] [CrossRef] [PubMed]
- Pinciroli, M.; Aphalo, P.; Nardo, A.E.; Añón, M.C.; Quiroga, A.V. Broken rice as a potential functional ingredient with inhibitory activity of renin and angiotensin-converting enzyme (ACE). Plant Food Hum. Nutr. 2019, 74, 405–413. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Velásquez-Sánchez, M.; Aguilar-Galvez, A.; Campos, D. In vitro antioxidant and angiotensin I-converting enzyme inhibitory properties of enzymatically hydrolyzed quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus) proteins. Cereal Chem. 2020, 97, 949–957. [Google Scholar] [CrossRef]
- Connolly, A.; O’Keeffe, M.B.; Piggott, C.O.; Nongonierma, A.B.; FitzGerald, R.J. Generation and identification of angiotensin converting enzyme (ACE) inhibitory peptides from a brewers’ spent grain protein isolate. Food Chem. 2015, 176, 64–71. [Google Scholar] [CrossRef]
- Betancur-Ancona, D.; Dávila-Ortiz, G.; Chel-Guerrero, L.A.; Torruco-Uco, J.G. ACE-I inhibitory activity from Phaseolus lunatus and Phaseolus vulgaris peptide fractions obtained by ultrafiltration. J. Med. Food 2015, 18, 1247–1254. [Google Scholar] [CrossRef]
- Shi, A.; Liu, H.; Liu, L.; Hu, H.; Wang, Q.; Adhikari, B. Isolation, purification and molecular mechanism of a peanut protein-derived ACE-inhibitory peptide. PLoS ONE 2014, 9, e111188. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela-García, P.; Bobadilla, N.A.; Ramírez-González, V.; León-Villanueva, A.; Lares-Asseff, I.A.; Valdez-Ortiz, A.; Medina-Godoy, S. Antihypertensive effect of protein hydrolysate from azufrado beans in spontaneously hypertensive rats. Cereal Chem. 2017, 94, 117–123. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M.; O’Shea, N.; Gallagher, E.; Lafarga, T. Predicted release and analysis of novel ACE-I, renin, and DPP-IV inhibitory peptides from common oat (Avena sativa) protein hydrolysates using in silico analysis. Foods 2017, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Muniraj, N.; Siddharth, S.; Sharma, D. Bioactive compounds: Multi-targeting silver bullets for preventing and treating breast cancer. Cancers 2019, 11, 1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, P.; Lu, M.; Petersen, S.; Ashkenazi, A. Chapter Five–Apoptosis initiation through the cell-extrinsic pathway. In Methods in Enzymology; Ashkenazi, A., Yuan, J., Wells, J.A., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 544, pp. 99–128. [Google Scholar]
- Shi, J.-H.; Sun, S.-C. Tumor necrosis factor receptor-associated factor regulation of nuclear factor κB and mitogen-activated protein kinase pathways. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Z.; Zhang, X.; Yu, P.; Chen, X.; Lu, P.; Li, M.; Liu, X.; Li, Z.; Wei, F.; Wang, K.; et al. Cinobufagin induces cell cycle arrest at the G2/M phase and promotes apoptosis in malignant melanoma cells. Front. Oncol. 2019, 9, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [Green Version]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Menon, D.B.; Gopalakrishnan, V. Terpenoids isolated from the shoot of Plectranthus hadiensis induces apoptosis in human colon cancer cells via the mitochondria-dependent pathway. Nutr. Cancer 2015, 67, 697–705. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, K.; Lee, D.H.; Shin, S.Y.; Yong, Y.; Lee, Y.H. Anti-invasive effect of β-myrcene, a component of the essential oil from Pinus koraiensis cones, in metastatic MDA-MB-231 human breast cancer cells. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 563–569. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Lampri, E.; Fitsiou, E.; Vasileiadis, S.; Vamvakias, M.; Bardouki, H.; Goussia, A.; Malamou-Mitsi, V.; Panayiotidis, M.I. Dietary mastic oil extracted from Pistacia lentiscus var. chia suppresses tumor growth in experimental colon cancer models. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, H.; Song, G.; Lim, W. Stigmasterol causes ovarian cancer cell apoptosis by inducing endoplasmic reticulum and mitochondrial dysfunction. Pharmaceutics 2020, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Kangsamaksin, T.; Chaithongyot, S.; Wootthichairangsan, C.; Hanchaina, R.; Tangshewinsirikul, C.; Svasti, J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS ONE 2017, 12, e0189628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Sala, A.; Attanzio, A.; Tesoriere, L.; Garcia-Llatas, G.; Barberá, R.; Cilla, A. Apoptotic effect of a phytosterol-ingredient and its main phytosterol (β-sitosterol) in human cancer cell lines. Int. J. Food Sci. Nutr. 2019, 70, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Farahmandfar, R.; Esmaeilzadeh Kenari, R.; Asnaashari, M.; Shahrampour, D.; Bakhshandeh, T. Bioactive compounds, antioxidant and antimicrobial activities of Arum maculatum leaves extracts as affected by various solvents and extraction methods. Food Sci. Nutr. 2019, 7, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Shashirekha, M.N.; Mallikarjuna, S.E.; Rajarathnam, S. Status of bioactive compounds in foods, with focus on fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 1324–1339. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Lampe, J.W. Spicing up a vegetarian diet: Chemopreventive effects of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 579S–583S. [Google Scholar] [CrossRef] [Green Version]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef] [Green Version]
- Wahyudha, D.; Mardawati, E.; Setyowati, E.; Dahlan, A.; Balia, R. The comparative study of the fruit and leaf extract of Ficuslyrata Warb on antibacterial activities. IOP Conf. Ser. Mater. Sci. Eng. 2018, 420, 012077. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Kim, J.-D.; Shin, J. Green tea seed isolated saponins exerts antibacterial effects against various strains of Gram positive and Gram negative bacteria, a comprehensive study in vitro and in vivo. Evid-Based Compl. Alt. Med. 2019, 2018, 3486106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Chen, J.; Xiao, A.; Liu, L. Antibacterial activity of polyphenols: Structure-activity relationship and influence of hyperglycemic condition. Molecules 2017, 22, 1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkan, G.; Sagdic, O.; Baydar, N.; Kurumahmutoglu, Z. Antibacterial activities and total phenolic contents of grape pomace extracts. J. Sci. Food Agric. 2004, 84, 1807–1811. [Google Scholar] [CrossRef]
- Yi, S.-M.; Zhu, J.-L.; Fu, L.-L.; Li, J.-R. Tea polyphenols inhibit Pseudomonas aeruginosa through damage to the cell membrane. Int. J. Food Microbiol. 2010, 144, 111–117. [Google Scholar] [CrossRef]
- Adnan, S.-N.-A.; Ibrahim, N.; Yaacob, W.A. Disruption of methicillin-resistant Staphylococcus aureus protein synthesis by tannins. Germs 2017, 7, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2012, 168, 1059–1073. [Google Scholar] [CrossRef] [Green Version]
- Yoda, Y.; Hu, Z.-Q.; Shimamura, T.; Zhao, W.-H. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure-activity relationship study of flavonoids as inhibitors of E. Coli by membrane interaction effect. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.d.C. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Chu, S.; Hagerman, A.; Lorigan, G. Probing the interaction of polyphenols with lipid bilayers by solid-state NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 6783–6789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tomiyama, D.; Yui, K.; Katsuki, M.; Ikeda, K.; Nonaka, A.; Miyamoto, T. Mechanism for the antibacterial action of epigallocatechin gallate (EGCg) on Bacillus subtilis. Biosci. Biotechnol. Biochem. 2015, 79, 845–854. [Google Scholar] [CrossRef]
- Gordon, N.C.; Wareham, D.W. Antimicrobial activity of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against clinical isolates of Stenotrophomonas maltophilia. Int. J. Antimicrob. Ag. 2010, 36, 129–131. [Google Scholar] [CrossRef]
- Cherubin, P.; Garcia, M.C.; Curtis, D.; Britt, C.B.T.; Craft, J.W., Jr.; Burress, H.; Berndt, C.; Reddy, S.; Guyette, J.; Zheng, T.; et al. Inhibition of cholera toxin and other AB toxins by polyphenolic compounds. PLoS ONE 2016, 11, e0166477. [Google Scholar] [CrossRef] [Green Version]
- Castillo, S.; Heredia, N.; García, S. 2(5H)-Furanone, epigallocatechin gallate, and a citric-based disinfectant disturb quorum-sensing activity and reduce motility and biofilm formation of Campylobacter jejuni. Folia Microbiol. 2015, 60, 89–95. [Google Scholar] [CrossRef]
- Parvez, A.K.; Saha, K.; Rahman, J.; Munmun, R.A.; Rahman, A.; Dey, S.K.; Rahman, S.; Islam, S.; Shariare, M.H. Antibacterial activities of green tea crude extracts and synergistic effects of epigallocatechingallate (EGCG) with gentamicin against MDR pathogens. Heliyon 2019, 5, e02126. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Kaihatsu, K.; Nishino, K.; Ogawa, M.; Kato, N.; Yamaguchi, A. Antibacterial and antifungal activities of new acylated derivatives of epigallocatechin gallate. Front. Microbiol. 2012, 16, 53. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; Kim, J.H.; Lee, C.K.; Oh, C.H.; Song, H.J. The antimicrobial activity of epigallocatehin-3-gallate and green tea extracts against Pseudomonas aeruginosa and Escherichia coli isolated from skin wounds. Ann. Dermatol. 2014, 26, 564–569. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, S.; Saei, H.D.; Ahmadi, M.; Salehi, T.Z. Antimicrobial effect of licochalcone A and epigallocatechin-3-gallate against Salmonella typhimurium isolated from poultry flocks. Iran. J. Microbiol. 2018, 10, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Vásquez, M.J.; Valenzuela-Buitimea, E.L.; Plascencia-Jatomea, M.; Encinas-Encinas, J.C.; Rodríguez-Félix, F.; Sánchez-Valdes, S.; Rosas-Burgos, E.C.; Ocaño-Higuera, V.M.; Graciano-Verdugo, A.Z. Functionalization of chitosan by a free radical reaction: Characterization, antioxidant and antibacterial potential. Carbohydr. Polym. 2017, 155, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Lagha, A.B.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum. Sci. Rep. 2017, 7, 44815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagha, A.B.; Groeger, S.; Meyle, J.; Grenier, D. Green tea polyphenols enhance gingival keratinocyte integrity and protect against invasion by Porphyromonas gingivalis. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef]
- Quiñones, B.; Massey, S.; Friedman, M.; Swimley, M.S.; Teter, K. Novel cell-based method to detect Shiga toxin 2 from Escherichia coli O157:H7 and inhibitors of toxin activity. Appl. Environ. Microbiol. 2009, 75, 1410–1416. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.; Kumar, A.; Kumar, P.; Mishra, D. Antimicrobial properties of black grape (Vitis vinifera L.) peel extracts against antibiotic-resistant pathogenic bacteria and toxin producing molds. Indian J. Pharmacol. 2015, 47, 663–667. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.; Taylor, M.; Zhao, M.; Cherubin, P.; Geden, S.; Ray, S.; Francis, D.; Teter, K. Grape extracts inhibit multiple events in the cell biology of cholera intoxication. PLoS ONE 2013, 8, e73390. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; He, C.; Song, L.; Li, T.; Cui, S.; Zhang, L.; Jia, Y. Antimicrobial activity and mechanism of Larch bark procyanidins against Staphylococcus aureus. Acta Biochim. Biophys. Sin. 2017, 49, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Biancalani, C.; Cerboneschi, M.; Tadini-Buoninsegni, F.; Campo, M.; Scardigli, A.; Romani, A.; Tegli, S. Global analysis of type three secretion system and quorum sensing inhibition of Pseudomonas savastanoi by polyphenols extracts from vegetable residues. PLoS ONE 2016, 11, e0163357. [Google Scholar] [CrossRef]
- Rémy, B.; Mion, S.; Plener, L.; Elias, M.; Chabrière, E.; Daudé, D. Interference in bacterial quorum sensing: A biopharmaceutical perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, X.; Zhang, F.; Feng, L.; Li, J. Inhibition of quorum sensing, biofilm, and spoilage potential in Shewanella baltica by green tea polyphenols. J. Microbiol. 2015, 53, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, H.; Chu, X.; Lou, T.; Zhang, N.; Zhang, B.; Chu, W. Tea polyphenols inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances resistance to Klebsiella pneumoniae infection in Caenorhabditis elegans model. Microb. Pathog. 2020, 147, 104266. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix tetrasperma impair virulence and inhibit quorum sensing of Pseudomonas aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Yifeng, D.; Wang, H.; Liu, W.; Zhuang, X.; Chu, W. Tea polyphenols as an antivirulence compound disrupt quorum-sensing regulated pathogenicity of Pseudomonas aeruginosa. Sci. Rep. 2015, 5, 16158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carraro, L.; Fasolato, L.; Montemurro, F.; Martino, M.E.; Balzan, S.; Servili, M.; Novelli, E.; Cardazzo, B. Polyphenols from olive mill waste affect biofilm formation and motility in Escherichia coli K-12. Microb. Biotechnol. 2014, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Pande, G.S.J.; Baruah, K.; Bossier, P. The apparent quorum-sensing inhibitory activity of pyrogallol is a side effect of peroxide production. Antimicrob. Agents Chemother. 2013, 57, 2870–2873. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.-W.; Yuan, G.-Q.; Li, Q.-q.; Lin, W. Antibacterial mechanisms of methyl gallate against Ralstonia solanacearum. Australas. Plant Pathol. 2014, 43, 1–7. [Google Scholar] [CrossRef]
- Hossain, M.A.; Park, N.-H.; Mechesso, A.F.; Birhanu, B.; Kang, J.; Reza, M.A.; Suh, J.-W.; Park, S.-C. Impact of phenolic compounds in the acyl homoserine lactone-mediated quorum sensing regulatory pathways. Sci. Rep. 2017, 7, 10618. [Google Scholar] [CrossRef] [Green Version]
- Westfall, S.; Lomis, N.; Prakash, S. A novel polyphenolic prebiotic and probiotic formulation have synergistic effects on the gut microbiota influencing Drosophila melanogaster physiology. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]


| Terpenes (Classification) | Amounts of Carbons | Isoprene Units |
|---|---|---|
| Hemiterpenoids | 5 | 1 |
| Monoterpenoids | 10 | 2 |
| Sesquiterpenoids | 15 | 3 |
| Diterpenoids | 20 | 4 |
| Sesterterpenoids | 25 | 5 |
| Triterpenoids | 30 | 6 |
| Tetraterpenoids | 40 | 8 |
| Politerpenoids | >40 | 8 |
| Source | Remarks Enzyme Aided Extraction Processes | Effects on Variable Responses | Reference |
|---|---|---|---|
| Plum (Prunus Domestica L.) seed waste | Protein extracts obtained by using four different enzymes: alcalase, thermolysin, flavourzyme, and protease P. | Alcalase was the enzyme yielding the extracts with the highest ABTS radical scavenging and lipid peroxidation inhibition capacities. Highest ACE inhibitory capacity was observed when using Thermolysin and alcalase | [127] |
| Walnut (Juglans regia L.) kernels | Enzymatic hydrolysate of walnut seed proteins was prepared by incubation with chymotrypsin, trypsin, and microbial proteinase K. | Walnut peptides have substantially higher antioxidant activity than intact proteins. Highest antioxidant and anticancer activity were exhibited by peptides produced with chymotrypsin | [128] |
| Cauliflower (Brassica oleracea L. var. botrytis) waste leaves and stem | By-product protein pellet was hydrolyzed by alcalase and trypsin | Compared to trypsin, the use of alcalase improved the antioxidant activity of hydrolysates while maintaining the ACE-inhibitor activity. | [129] |
| Partially defatted Riceberry bran | The effect of enzyme type (alcalase, flavourzyme and neutrase) and hydrolysis time (2, 4 and 6 h) on protein yield, total phenolic content (TPC), and antioxidant activities (ABTS and FRAP) was investigated. | The enzyme type significantly (P < 0.05) affected the properties of peptides, whereas the hydrolysis time had no significant effect (P ≥ 0.05). Flavourzyme was the most effective to increase protein yield, TPC, and antioxidant activities compared to alcalase and neutrase. | [130] |
| Limonia acidissima seed | Three-level three-factor Box Behnken Design was employed to predict the optimal hydrolysis conditions of L. acidissima using pepsin. Evaluated independent variables were: pH, enzyme substrate ratio, and time. | The three evaluated factors (pH, enzyme substrate ratio, and time) exerted a significant effect (P < 0.05) on degree of hydrolysis (DH). The optimal conditions for L acidissima seed proteins were pH 2, enzyme substrate ratio of 2.5% (w/w), and hydrolysis time of 42.41 min. Under these conditions, a DH of 36.59% was achieved. | [131] |
| Food waste sesame bran (Sesamum indicum L.) | Three different strategies of extraction were applied to obtain peptides from sesame bran: conventional alkaline extraction (as a control), microwave-assisted extraction (independent variables: time and temperature), and combined microwave-assisted enzymatic extraction, using alcalase (independent variables: enzyme concentration, pH, and time of hidrolysys). RSM was used to optimize the processes. | All the independent variables had a significant effect on all of the responses at a level of P < 0.001. The highest protein yield was obtained by combined microwave-assisted enzymatic extraction (91.7%). Total phenolic compound results were in accordance with protein yields and resulted as 3.45, 4.20, and 8.04 mg GAE/g for alkaline, microwave-assisted, and combined extraction, respectively. Microwave-assisted enzymatic extraction was proven to be more efficient than other techniques on the extraction of protein and bioactive compounds. | [9] |
| Technique | Main Parameters/Factors | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Maceration | Solvents, solid-to-solvent ratio, agitation, temperature, time of extraction | Simple method, it does not require expensive equipment, the use of mild temperature conditions allows to extract thermolabile compounds | Use of great volumes of organic solvents, prolonged time of extraction, it requires an adittional step of separation (evaporation/concentration) | [163] |
| Hydrodystillation | Particle size, time of extraction, solid-to-solvent ratio | It does not require organic solvents (water can be used) | Thermolabile compounds can be degraded, prolonged time of extraction | [100] |
| Ultrasonic-assisted extraction | Frequency, extraction time, temperature | Higher recovery of targeted compounds, | Requires specific equipment, might degrade unstable compounds (carotenoids) | [164] |
| Microwave-assisted extraction | Frequency, time of microwave, moisture content, particle size, solid-to-liquid ratio, temperature | Reduces extraction time and solvent consumption, shows a higher performance in the recovery of bioactive compounds | Requires specific equipment, increases in temperature can cause the generation of undesirable compounds (hydroxymethylfurfural) | [165] |
| Pressurized liquids | Temperature, pressure | Faster and efficient extractions, requires smaller volumes of solvents than traditional methods | Might not suitable for thermolabile compounds (the high temperatures can degrade the chemical structure and functional activity of targeted compounds) | [142,166] |
| Supercritical fluids | Temperature, pressure, co-solvent, time, particle size | More efficient extraction, doesn’t require additional steps of separation, more specificity towards the targeted compounds, maintains the chemical structure and functional activity of targeted compounds | Requires expensive equipment, although it is already used in industrial processes, such as coffee decaffeination | [155] |
| Waste | Scientific Name | Plant Part | Method of Identification | Compounds | Reference |
|---|---|---|---|---|---|
| Pomegranate (15 different varieties) | Punica granatum L. | Peel decoctions | HPLC-DAD and HPLC-MS | Punicalagin, cyanidin-3,5-O-diglucoside, delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, pelargonidin-3-O-glucoside | [188] |
| Pomegranate | Punica granatum L. | Peel | HPLC-DAD-ESI-MS | Protocatechuic acid, cis-p-coumaric acid, trans-p-coumaric acid, vanillic acid, gallic acid, hydroxygallic acid, hydroxycaffeic acid, vanillic acid hexoside, caffeic acid hexoside, ferulic acid hexoside, 5-O-caffeoylquinic acid | [189] |
| Pomegranate | Punica granatum L. | Peel | HPLC-DAD and LC-MS/MS | Punicalin, punicalagin, pedunculagin, castalagin derivate, ellagic acid hexoside, ellagic acid pentoside, ellagic acid deoxyhexoside, ellagic acid, gallic acid | [20] |
| Pomegranate | Punica granatum L. | Peel | HPLC | Punicalagin, pyrogallol, ellagic acid, gallic acid, protocatechuic acid, chlorogenic acid, p-hydroxybenzoic acid, cinnamic acid, hesperidin, quercitrin | [190] |
| Passion fruit | Passiflora edulis | Peel | HPLC | Caffeic acid and isoorientin | [191] |
| Naranjilla | Solanum quitoense | Peel | UHPLC-HRMS | Chlorogenic acid, p-coumaric acid, gallic acid, rutin hydrate, taxifolin | [192] |
| Cape gooseberry | Physalis peruviana | Peel | Chlorogenic acid, gallic acid, polydatin, rutin hydrate, | ||
| Tamarillo | Cyphomandra betaceae | Peel | Chlorogenic acid, p-coumaric acid, ferulic acid, rutin hydrate, sinapic acid, syringaldehyde, taxifolin | ||
| Poro poro | Passiflora pinnatistipula | Peel | Trans-cinnamic acid, p-coumaric acid, (−)-epicatechin, ferulic acid, gallic acid, polydatin, rutin hydrate, sinapic acid, taxifolin | ||
| Curuba | Passiflora tripartita | Peel | (+)-catechin, p-coumaric acid, ferulic acid, polydatin, sinapic acid, vanillic acid | ||
| Sweet granadilla | Passiflora ligularis | Peel | (+)-catechin, chlorogenic acid, p-coumaric acid, ferulic acid, gallic acid, homogentisic acid, polydatin, sinapic acid, vanillic acid | ||
| Araticum | Annona crassiflora Mart. | Peel | HPLC-ESI-MS/MS | Catechin, epicatechin, rutin, quercetin, protocatechuic acid, gentisic acid, chlorogenic acid, p-coumaric acid, ferulic acid | [193] |
| Longan | Dimocarpus longan | Peel | HPLC-DAD | Ellagic acid, corilagin, gallic acid, o-coumaric acid, ferulic acid, chlorogenic acid, quercetin, kaempferol | [194] |
| Red grape | Vitis vinifera cv. Sangiovese | Peel | HPLC-DAD | Gallic acid, (+)-catechin, caffeic acid, (−)-epicatechin, rutin, trans-resveratrol, quercetin | [195] |
| Red grape | Vitis vinifera (cv. Sangiovese and Montepulciano) | Pomace | HPLC-DAD | Quercetin, rutin, cis-piceid, catechin, epicatechin, epigallocatechin gallate, epicatechin gallate, epigallocatechin, gallic acid, vanillin, vanillic acid | [196] |
| Red grape | Cultivars Chambourcin (hybrid), Merlot (Vitis vinífera), Norton (Vitis aestivalis), Petit Verdot (V. vinifera), Tinta Cao (V. vinifera) | Pomace | HPLC-DAD | Malvidin chloride, gallic acid, catechin, delphinidin chloride, caffeic acid, cyanidin chloride, p-coumaric acid, epicatechin gallate, rutin, quercetin-3-O-glucoside, myricetin, resveratrol, quercetin hydrate, kaempferol | [197] |
| Pistachios | Pistacia vera L. | Shell skin | RP-HPLC-DAD | Gallic acid, protocatechuic acid, (+)-catechin, p-hydroxybenzoic acid, caffeic acid, (−)-epicatechin, syringic acid, p-coumaric acid, hesperidin, quercetin, apigenin | [198] |
| White grape | Vitis vinifera | Peel | UPLC-DAD-MS and MALDI-TOF-MS for proanthocyanidins | Catechin, epicatechin, quercetin-3-O-glucuronide, quercetin-3-O-glucoside, quercetin-3-O-rhamnoside, kaempferol-3-O-galactoside, kaempferol-3-O-glucuronide, kaempferol-3-O-glucoside, quercetin, kaempferol, oligomeric proanthocyanidins | [199] |
| Jackfruit | Arpus heterophyllus Lam. | Peel | HPLC-QTOF-MS/MS | Cis-3-caffeoylquinic acid, trans-3-caffeoylquinic acid, esculetin-O-hexoside, esculetin-C-hexoside, 3,4-dihydroxybenzoic methyl ester-C-dihexoside, esculetin-hexoylpentoside, feruloylglucoside, cis-3-caffeoylquinic acid, caffeoylglucoside, cis-5-caffeoylquinic acid, trans-5-caffeoylquinic acid, trans-4-caffeoylquinic acid, procyanidin B, epicatechin, dihydroquercetin, prenyl-O-naringenin, prenylgenistein, pentenylnaringenin, hexenyl-5,7,4’-trihydroxyflavan | [200] |
| Waste | Source or Matrix | Enzymes or Hydrolysis Conditions | Peptide Sequence a (IC50) b | Reference |
|---|---|---|---|---|
| Proteins from oils manufacturing | Coconut cake albumin | Alcalase, flavourzyme, pepsin, and trypsin (sequential hydrolysis) | KAQYPYV (37.06 mM) KIIIYN (58.72 mM) KILIYG (53.31 mM) | [32] |
| Walnut (Juglans regia L.) meal | Pepsin | YEP (0.29 µM) | [215] | |
| Rapeseed meal | Alcalase | LY (0.11 mM, 1.87 mM *) TF (0.81 mM) RALP (0.65 mM, 0.97 mM *) GHS (1.74 mM *) | [27,216,217] | |
| Sweet almond (Prunus armeniaca L.) | Alcalase | MHTDD (7.52 µg/mL) GHTDD (43.18 µg/mL) | [214] | |
| Vegetable waste | Cauliflower (Brassica oleracea L. var. botrytis) leaves and stems | Alcalase | APYDPDWYYIR (2.59 μM) SKGFTSPLF (15.26 µM) | [129] |
| VT (31.30 μM) | [218] | |||
| Asparagus (Asparagus officinalis) | Alcalase | PDWFLLL (1.76 μM) ASQSIWLPGWL (4.02 μM) | [219] | |
| Broccoli (Brassica oleracea var. italica cv. Lvxiong 90) | Chymotrypsin | IPPAYTK (23.5 μM) LVLPGELAK (184.0 μM) TFQGPPHGIQVER (3.4 μM) LVLPGE (13.5 μM) LAK (48.0 μM) | [12] | |
| Seeds and others waste from peeler/pitting process on fruits | Peach seed proteins | Thermolysin | IYSPH (39.5 μM) | [30] |
| Olive (Olea europaea L.) seed proteins | Thermolysin | LLPSY (39.9 µM) | [220] | |
| Cereals, pseudocereals, and legumes industry | Cherry (Prunus Cerasus L.) by products | Thermolysin | n.r. (0.31 mg/mL) | [182] |
| Plum (Prunus domestica L.) seed | Thermolysin | n.r. (22.8 µg/mL) | [26] | |
| Date (Phoenix dactylifera L.) seed flour | Alcalase and Thermolysin | n.r. (0.53 mg/mL) | [181] | |
| Tomato seeds | Bacillus subtilis Fermentation | DGVVYY (2 µM) | [29] | |
| Wheat bran | Alcalase | NL, QL, FL, HAL, AAVL, AKTVF, TPLTR | [221] | |
| Quinoa (Chenopodium quinoa) | Neutrase | n.r. (0.08 mg/mL) | [223] | |
| Kiwicha (Amaranthus caudatus) | Alcalase-Neutrase (sequential hydrolysis) | n.r. (0.29 mg/mL) | ||
| Broken rice | Pepsin | n.r. (0.87 mg/mL) | [222] | |
| Rice (Oyaza sativa) bran | Tripsyn | YSK (76 µM) | [31] | |
| Azufrado beans (Phaseolus vulgaris L.) | Alcalase | KFPWVK, GADFRKK, PQSPCKRVNRHS | [227] | |
| Lima bean (Phaseolus lunatus) | Alcalase Flavourzyme | n.r. (30.3 µg/mL) n.r. (51.8 µg/mL) | [225] | |
| Brewing industry: Brewers’ spent grain | Peanut (Arachis hypogaea) | Alcalase | KLYMRP (6.42 µM) | [226] |
| Barley | Alcalase, Corolase PP, Flavourzyme and Promod 144MG | IVY (80.4 μM) ILDL (96.4 μM) | [224] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizárraga-Velázquez, C.E.; Leyva-López, N.; Hernández, C.; Gutiérrez-Grijalva, E.P.; Salazar-Leyva, J.A.; Osuna-Ruíz, I.; Martínez-Montaño, E.; Arrizon, J.; Guerrero, A.; Benitez-Hernández, A.; et al. Antioxidant Molecules from Plant Waste: Extraction Techniques and Biological Properties. Processes 2020, 8, 1566. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8121566
Lizárraga-Velázquez CE, Leyva-López N, Hernández C, Gutiérrez-Grijalva EP, Salazar-Leyva JA, Osuna-Ruíz I, Martínez-Montaño E, Arrizon J, Guerrero A, Benitez-Hernández A, et al. Antioxidant Molecules from Plant Waste: Extraction Techniques and Biological Properties. Processes. 2020; 8(12):1566. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8121566
Chicago/Turabian StyleLizárraga-Velázquez, Cynthia E., Nayely Leyva-López, Crisantema Hernández, Erick Paul Gutiérrez-Grijalva, Jesús A. Salazar-Leyva, Idalia Osuna-Ruíz, Emmanuel Martínez-Montaño, Javier Arrizon, Abraham Guerrero, Asahel Benitez-Hernández, and et al. 2020. "Antioxidant Molecules from Plant Waste: Extraction Techniques and Biological Properties" Processes 8, no. 12: 1566. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8121566