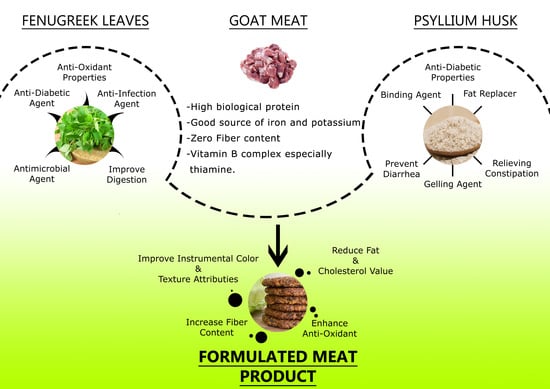
Optimum Additive Composition to Minimize Fat in Functional Goat Meat Nuggets: A Healthy Red Meat Functional Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Experiment: Formulation of Experimental Functional Meat Nuggets
2.2. Quality Parameter Protocols
2.3. In Vitro Human Digestion Model
2.4. Thiobarbituric Acid-Reacting Substances (TBARS) Lipid Peroxidation Test
2.5. Response Surface Optimization
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Smet, S.; Vossen, E. Meat: The balance between nutrition and health. A review. Meat Sci. 2016, 120, 145–156. [Google Scholar] [CrossRef]
- Mazhangara, I.R.; Chivandi, E.; Mupangwa, J.F.; Muchenje, V. The potential of goat meat in the red meat industry. Sustainability 2019, 11, 3671. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.D.X.; Medeiros, A.N.; Oliveira, R.L.; de Araújo, G.G.L.; Queiroga, R.d.C.d.E.; Ribeiro, M.D.; Silva, T.M.; Bezerra, L.R.; Oliveira, R.L. Palm kernel cake from the biodiesel industry in goat kid diets. Part 2: Physicochemical composition, fatty acid profile and sensory attributes of meat. Small Rumin. Res. 2018, 165, 1–7. [Google Scholar] [CrossRef]
- Gopalan, C.; Ramasastri, B.V.; Balasubramanian, S.C. Foods and their nutrient content. In Nutritive Value of Indian Foods; National Institute of Nutrition, ICMR: Hyderabad, India, 2004; pp. 27–29. [Google Scholar]
- Madruga, M.S.; Medeiros, E.J.L.d.; Sousa, W.H.d.; Cunha, M.d.G.G.; Pereira Filho, J.M.; Queiroga, R.d.C.R.d.E. Chemical composition and fat profile of meat from crossbred goats reared under feedlot systems. Rev. Bras. de Zootec. 2009, 38, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Al-Shaar, L.; Satija, A.; Wang, D.D.; Rimm, E.B.; Smith-Warner, S.A.; Stampfer, M.J.; Hu, F.B.; Willett, W.C. Red meat intake and risk of coronary heart disease among US men: Prospective cohort study. BMJ 2020, 371. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Meat consumption and risk of colorectal cancer: A meta-analysis of prospective studies. Int. J. Cancer 2006, 119, 2657–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesalski, H.K. Meat as a component of a healthy diet-are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005, 70, 509–524. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fiber in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fiber enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Chao, A.; Thun, M.J.; Connell, C.J.; McCullough, M.L.; Jacobs, E.J.; Flanders, W.D.; Rodriguez, C.; Sinha, R.; Calle, E.E. Meat consumption and risk of colorectal cancer. JAMA 2005, 293, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Hogg, N. Red meat and colon cancer: Heme proteins and nitrite in the gut. A commentary on “diet-induced endogenous formation of nitroso compounds in the GI tract”. Free Radic. Biol. Med. 2007, 43, 1037. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Gillespie, C.; Rosenbaum, E.H.; Jenson, C. A rapid food screener to assess fat and fruit and vegetable intake. Am. J. Prev. Med. 2000, 18, 284–288. [Google Scholar] [CrossRef]
- Kausar, T.; Kausar, M.A.; Azad, Z. Improving the quality and shelf life of goat meat patties with herb and husk incorporation. Biochem. Cell. Arch. 2018, 18, 1569–1576. [Google Scholar]
- Kausar, T.; Hanan, E.; Ayob, O.; Praween, B.; Azad, Z. A review on functional ingredients in red meat products. Bioinformation 2019, 15, 358. [Google Scholar] [CrossRef]
- Robel, C.; Ktenioudaki, A.; Gallagher, E. Inulin and oligofructose as fat and sugar substitutes in quick breads (scones): A mixture design approach. Eur. Food Res. Technol. 2011, 233, 167. [Google Scholar]
- Nardoia, M.; Ruiz-Capillas, C.; Herrero, A.M.; Pintado, T.; Jimnez-Colmenero, F.; Chamorro, S.; Brenes, A. Effect of added grape seed and skin on chicken thigh patties during chilled storage. Int. J. Food Nutr. Sci. 2017, 4, 67–73. [Google Scholar]
- Das, A.K.; Rajkumar, V.; Verma, A.K. Bael pulp residue as a new source of antioxidant dietary fiber in goat meat nuggets. J. Food Process. Preserv. 2015, 39, 1626–1635. [Google Scholar] [CrossRef] [Green Version]
- Mehta, N.; Ahlawat, S.S.; Sharma, D.P.; Dabur, R.S.; Yadav, S. Optimization and quality evaluation of dietary fiber rich chicken meat rolls incorporated with psyllium husk. Fleischwirtsch. Int. 2016, 3, 65–70. [Google Scholar]
- Madane, P.; Das, A.K.; Pateiro, M.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Barba, F.J.; Shewalkar, A.; Maity, B.; Lorenzo, J.M. Drumstick (Moringa oleifera) flower as an antioxidant dietary fiber in chicken meat nuggets. Foods 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Strategies for incorporation of chia (Salvia hispanica L.) in frankfurters as a health-promoting ingredient. Meat Sci. 2016, 114, 75–84. [Google Scholar] [CrossRef]
- Fang, Z.; Lin, P.; Ha, M.; Warner, R.D. Effects of incorporation of sugarcane fiber on the physicochemical and sensory properties of chicken sausage. Int. J. Food Sci. Technol. 2019, 54, 1036–1044. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Thorat, P.R.; Manjunatha, M.; Anurag, R.K. Comparative antioxidant effect of aqueous extracts of curry leaves, fenugreek leaves and butylated hydroxytoluene in raw chicken patties. J. Food Sci. Technol. 2012, 49, 781–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitev, A.; Kuzelov, A.; Josevska, E. Influence of Goji berries on oxidative changes, microbiological status and chemical properties of sausages. Agric. Sci. Technol. 2018, 10, 70–73. [Google Scholar] [CrossRef]
- Pereira, D.; Pinheiro, R.S.; Heldt, L.F.S.; Moura, C.d.; Bianchin, M.; Almeida, J.d.F.; Reis, A.l.S.d.; Ribeiro, I.S.; Haminiuk, C.W.I.; Carpes, S.T. Rosemary as natural antioxidant to prevent oxidation in chicken burgers. Food Sci. Technol. 2017, 37, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, J.M.; Munekata, P.E.S. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac. J. Trop. Biomed. 2016, 6, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Bhat, Z.F.; Bhat, H. Functional meat products: A review. Int. J. Meat Sci. 2011, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Mogra, R. Psyllium (Plantago ovata) husk: A wonder food for good health. Int. J. Sci. Res. 2013, 4, 1581–1585. [Google Scholar]
- Punna, R.; Rao Paruchuri, U. Effect of maturity and processing on total, insoluble and soluble dietary fiber contents of Indian green leafy vegetables. Int. J. Food Sci. Nutr. 2004, 55, 561–567. [Google Scholar] [CrossRef]
- Rodriguez, R.; Jimenez, A.; Fernandez-Bolanos, J.; Guillen, R.; Heredia, A. Dietary fiber from vegetable products as source of functional ingredients. Trends Food Sci. Technol. 2006, 17, 3–15. [Google Scholar] [CrossRef]
- Kendall, C.W.C. The health benefits of Psyllium. Can. J. Diet. Pract. Res. 2004, 65, A1. [Google Scholar]
- Sumayya, A.R.; Sivagami, S.; Nabeelah, A. Screening and biochemical quantification of phytochemicals in fenugreek (Trigonella foenum-graecum). Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 165–169. [Google Scholar]
- Franco, E.A.N.; Sanches-Silva, A.; Ribeiro-Santos, R.; de Melo, N.R. Psyllium (Plantago ovata Forsk): From evidence of health benefits to its food application. Trends Food Sci. Technol. 2020, 96, 166–175. [Google Scholar] [CrossRef]
- Hanafy, R.S.; Akladious, S.A. Physiological and molecular studies on the effect of gamma radiation in fenugreek (Trigonella foenum-graecum L.) plants. J. Genet. Eng. Biotechnol. 2018, 16, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.N.; Mat Noh, N.A.; Abdullah, E.N.; Yarmo, M.A.; Mat Piah, M.B.; Ku Bulat, K.H. Optimization of a protease extraction using a statistical approach for the production of an alternative meat tenderizer from Spondias cytherea roots. J. Food Process. Preserv. 2019, 43, e14192. [Google Scholar] [CrossRef]
- Louaer, M.; Zermane, A.; Larkeche, O.; Meniai, A.H. Experimental study and optimization of the extraction of Algerian date stones oil (Phoenix dactylifera L.) using supercritical carbon dioxide. J. Food Process Eng. 2020, 42, e13049. [Google Scholar] [CrossRef]
- Singh, J.S.; Koushal, S.; Kumar, A.; Vimal, S.R.; Gupta, V.K. Book review: Microbial inoculants in sustainable agricultural productivity-Vol. II: Functional application. Front. Microbiol. 2017, 7, 2105. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.N.; Liew, S.L.; Yarmo, M.A.; Said, M. Optimization of protease extraction from horse mango (Mangifera foetida Lour) kernels by a response surface methodology. Biosci. Biotechnol. Biochem. 2012, 76, 1438–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gok, V.; Akkaya, L.; Obuz, E.; Bulut, S. Effect of ground poppy seed as a fat replacer on meat burgers. Meat Sci. 2011, 89, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Moon, S.S.; Lee, S.J. In vitro effects of cooking methods on digestibility of lipids and formation of cholesterol oxidation products in pork. Korean J. Food Sci. Anim. Resour. 2014, 34, 280. [Google Scholar] [CrossRef] [Green Version]
- Versantvoort, C.H.M.; Oomen, A.G.; Van de Kamp, E.; Rompelberg, C.J.M.; Sips, A.n.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. [30] Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Zoulias, E.I.; Oreopoulou, V.; Tzia, C. Textural properties of low-fat cookies containing carbohydrate-or protein-based fat replacers. J. Food Eng. 2002, 55, 337–342. [Google Scholar] [CrossRef]
- Madane, P.; Das, A.K.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Shewalkar, A.; Maity, B. Dragon fruit (Hylocereus undatus) peel as antioxidant dietary fiber on quality and lipid oxidation of chicken nuggets. J. Food Sci. Technol. 2019, 57, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Ahlawat, S.S.; Sharma, D.P.; Yadav, S.; Arora, D. Development and quality evaluation of chicken patties incorporated with psyllium husk. Haryana Vet. 2013, 52, 6–11. [Google Scholar]
- Zargar, F.A.; Kumar, S.; Bhat, Z.F.; Kumar, P. Effect of pumpkin on the quality characteristics and storage quality of aerobically packaged chicken sausages. SpringerPlus 2014, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Jairath, G.; Sharma, D.P.; Dabur, R.S.; Singh, P.K.; Bishnoi, S. Standardization of corn starch as a fat replacer in buffalo calf meat sausages and its effect on the quality attributes. Indian J. Anim. Res. 2018, 52, 1521–1525. [Google Scholar] [CrossRef] [Green Version]
- Rokib, M.; Habib, M.; Hashem, M.A.; Ali, M.S. Value addition of low fat chicken sausage with rice and wheat flour. Bangladesh J. Anim. Sci. 2019, 48, 99–107. [Google Scholar] [CrossRef]
- Ham, Y.-K.; Hwang, K.-E.; Song, D.-H.; Kim, Y.-J.; Shin, D.-J.; Kim, K.-I.; Lee, H.-J.; Kim, N.-R.; Kim, C.-J. Lotus (Nelumbo nucifera) rhizome as an antioxidant dietary fiber in cooked sausage: Effects on physicochemical and sensory characteristics. Korean J. Food Sci. Anim. Resour. 2017, 37, 219. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Lairon, D.; Arnault, N.; Bertrais, S.; Planells, R.; Clero, E.; Hercberg, S.; Boutron-Ruault, M.-C. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am. J. Clin. Nutr. 2005, 82, 1185–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaafsma, G. Health claims, options for dietary fiber. Diet. Fiber Bioact. Carbohydr. Food Feed 2004, 27–38. [Google Scholar]
- Weickert, M.O.; Pfeiffer, A.F.H. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Manach, C.; Faulks, R.M.; Kroon, P.A. Absorption and metabolism of dietary plant secondary metabolites. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Wiley-Blackwell: Oxford, UK, 2006; pp. 303–351. [Google Scholar]
- Muraki, I.; Imamura, F.; Manson, J.E.; Hu, F.B.; Willett, W.C.; van Dam, R.M.; Sun, Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 2013, 347, f5001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, U.C.S.; Baquer, N.Z. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm. Biol. 2014, 52, 243–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, K.T. The potential of fenugreek (Trigonella foenum-graecum) as a functional food and nutraceutical and its effects on glycemia and lipidemia. J. Med. Food 2011, 14, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Alvarez-Parrilla, E.; De La Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Serrano, J.; Tabernero, M.; Arranz, S.; Diaz-Rubio, M.E.; Garcia-Diz, L.; Goni, I.; Saura-Calixto, F. Bioavailability of phenolic antioxidants associated with dietary fiber: Plasma antioxidant capacity after acute and long-term intake in humans. Plant Foods Hum. Nutr. 2009, 64, 102–107. [Google Scholar] [CrossRef]
- Metzler, B.U.; Mosenthin, R. A review of interactions between dietary fiber and the gastrointestinal microbiota and their consequences on intestinal phosphorus metabolism in growing pigs. Asian-Australas. J. Anim. Sci. 2008, 21, 603–615. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Jiang, H.; Nie, D. The role of short-chain fatty acids in orchestrating two types of programmed cell death in colon cancer. Autophagy 2011, 7, 235–237. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.; Sello, C.T.; Qin, G.-X.; Che, D.; Han, R. Does dietary fiber affect the levels of nutritional components after feed formulation? Fibers 2018, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Terpstra, A.H.M.; Lapre, J.A.; De Vries, H.T.; Beynen, A.C. Hypocholesterolemic effect of dietary psyllium in female rats. Ann. Nutr. Metab. 2000, 44, 223–228. [Google Scholar] [CrossRef]
- Rodriguez-Cabezas, M.E.; Galvez, J.; Camuesco, D.; Lorente, M.D.; Concha, A.; Martinez-Augustin, O.; Redondo, L.; Zarzuelo, A. Intestinal anti-inflammatory activity of dietary fiber (Plantago ovata seeds) in HLA-B27 transgenic rats. Clin. Nutr. 2003, 22, 463–471. [Google Scholar] [CrossRef]
- Rakita, S.; Spasevski, N.; Čolović, D.; Popović, S.; Ikonić, P.; Čolović, R.; Lević, J. The influence of laying hens’ diet enriched with omega-3 fatty acids, paprika and marigold on physical properties of eggs. J. Process. Energy Agric. 2016, 20, 58–62. [Google Scholar]




| GM | FL | PH | OFC | RMPFC | pH | MC | AC | CF | OA |
|---|---|---|---|---|---|---|---|---|---|
| 55 | 3 | 1 | 5.85 | 5.83 | 6.59 | 61.8 | 3.88 | 1.09 | 9 |
| 55 | 3 | 5 | 5 | 4.97 | 6.71 | 60.2 | 3.90 | 1.78 | 8 |
| 55 | 9 | 1 | 5 | 5.07 | 6.51 | 62.1 | 4.02 | 1.21 | 8 |
| 55 | 9 | 5 | 4.8 | 4.78 | 6.60 | 61.5 | 4.06 | 1.42 | 7 |
| 65 | 3 | 1 | 7 | 7.06 | 6.65 | 63.8 | 3.94 | 1.21 | 8 |
| 65 | 3 | 5 | 5.8 | 5.77 | 6.69 | 62.3 | 3.96 | 1.46 | 7 |
| 65 | 9 | 1 | 6.2 | 6.27 | 6.49 | 64.1 | 4.17 | 1.81 | 9 |
| 65 | 9 | 5 | 5.5 | 5.56 | 6.55 | 63.7 | 4.29 | 1.94 | 7 |
| 51.59 * | 6 | 3 | 4.3 | 4.31 | 6.62 | 60.7 | 3.74 | 1.19 | 8 |
| 68.40 ** | 6 | 3 | 6.1 | 6.01 | 6.58 | 63.9 | 4.10 | 1.48 | 8 |
| 60 | 0.95 * | 3 | 6.4 | 6.42 | 6.65 | 62.4 | 3.66 | 1.01 | 8 |
| 60 | 11.04 ** | 3 | 5.7 | 5.60 | 6.55 | 62.8 | 4.23 | 1.58 | 8 |
| 60 | 6 | 0 * | 6.6 | 6.48 | 6.47 | 64.3 | 3.65 | 1.07 | 9 |
| 60 | 6 | 6.3 ** | 5.2 | 5.21 | 6.74 | 60.7 | 3.83 | 1.91 | 7 |
| 60 | 6 | 3 | 6 | 5.96 | 6.67 | 63.7 | 3.79 | 1.70 | 8 |
| 60 | 6 | 3 | 5.9 | 5.96 | 6.67 | 63.3 | 3.76 | 1.55 | 8 |
| 60 | 6 | 3 | 5.8 | 5.96 | 6.68 | 63.8 | 3.78 | 1.59 | 7 |
| 60 | 6 | 3 | 6.1 | 5.96 | 6.66 | 63.9 | 3.77 | 1.58 | 8 |
| 60 | 6 | 3 | 6.1 | 5.96 | 6.67 | 63.7 | 3.79 | 1.59 | 7 |
| 60 | 6 | 3 | 6.1 | 5.96 | 6.68 | 63.5 | 3.78 | 1.58 | 8 |
| 60 | 6 | 3 | 5.8 | 5.96 | 6.68 | 63.6 | 3.78 | 1.57 | 7 |
| 60 | 6 | 3 | 5.8 | 5.96 | 6.67 | 63.8 | 3.78 | 1.59 | 8 |
| 60 | 6 | 3 | 6 | 5.96 | 6.67 | 63.7 | 3.79 | 1.58 | 8 |
| 60 | 6 | 3 | 6 | 5.96 | 6.68 | 63.8 | 3.78 | 1.57 | 8 |
| Term | Coef. | Adj SS | Adj MS | F-Value | P-Value |
|---|---|---|---|---|---|
| Constant () | −41.33 | 7.88454 | 0.87606 | 62.25 | 0.000 |
| Goat meat (g) | 1.492 | 3.28529 | 3.28529 | 233.44 | 0.000 |
| Fenugreek leaves (g) | −0.152 | 0.71591 | 0.71591 | 50.87 | 0.000 |
| Psyllium husk (g) | 0.341 | 2.06809 | 2.06809 | 146.95 | 0.000 |
| Overall Linear Coefficient Significance | 6.06929 | 2.02310 | 143.76 | 0.000 | |
| Goat meat (g)*goat meat (g) | −0.01131 | 1.29438 | 1.29438 | 91.98 | 0.000 |
| Fenugreek leaves (g)*fenugreek leaves (g) | 0.00199 | 0.00517 | 0.00517 | 0.37 | 0.554 |
| Psyllium husk (g)*psyllium husk (g) | −0.00740 | 0.01208 | 0.01208 | 0.86 | 0.370 |
| Overall Quadratic Coefficient Significance | 1.32036 | 0.44012 | 31.27 | 0.000 | |
| Goat meat (g)*fenugreek leaves (g) | −0.00042 | 0.00031 | 0.00031 | 0.02 | 0.884 |
| Goat meat (g)*psyllium husk (g) | −0.01063 | 0.09031 | 0.09031 | 6.42 | 0.024 |
| Fenugreek leaves (g)*psyllium husk (g) | 0.02369 | 0.16531 | 0.16531 | 11.75 | 0.004 |
| Overall, Two-Way Interaction Coefficient Significance | 0.25594 | 0.08531 | 6.06 | 0.007 | |
| Quadratic Response Equation (Equation (2)) | S | R2 | R2-(adj) | R2-(pred) | |
|---|---|---|---|---|---|
| Fat (%) = −41.33 + 1.492 − 0.152 + 0.341 − 0.01131 + 0.00199 − 0.00740 − 0.00042 * − 0.01063 * + 0.02396 * | 0.118630 | 97.56% | 95.99% | 92.99% | |
| Optimum Goat Meat (g) | Optimum Fenugreek Leaves (g) | Optimum Psyllium Husk (g) | |||
| Minimized fat content (%) predicted at optimum values | 3.87092 | 51.5910 | 5.23555 | 6.36359 | |
| Minimized fat content (%) observed at optimum values | 3.5 ± 0.3 | 51.6 | 5.2 | 6.3 | |
| Fat content (%) in control one | 4.9 ± 2 | 51.6 | - | - | |
| Fat content (%) in control two (weight-conserved nuggets) | 5.8 ± 2 | 63.1 | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, T.; Kausar, M.A.; Khan, S.; Haque, S.; Azad, Z.R.A.A. Optimum Additive Composition to Minimize Fat in Functional Goat Meat Nuggets: A Healthy Red Meat Functional Food. Processes 2021, 9, 475. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9030475
Kausar T, Kausar MA, Khan S, Haque S, Azad ZRAA. Optimum Additive Composition to Minimize Fat in Functional Goat Meat Nuggets: A Healthy Red Meat Functional Food. Processes. 2021; 9(3):475. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9030475
Chicago/Turabian StyleKausar, Tahreem, Mohd Adnan Kausar, Saif Khan, Shafiul Haque, and Z. R. Azaz Ahmad Azad. 2021. "Optimum Additive Composition to Minimize Fat in Functional Goat Meat Nuggets: A Healthy Red Meat Functional Food" Processes 9, no. 3: 475. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9030475