Low Molecular Weight Kappa-Carrageenan Based Microspheres for Enhancing Stability and Bioavailability of Tea Polyphenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
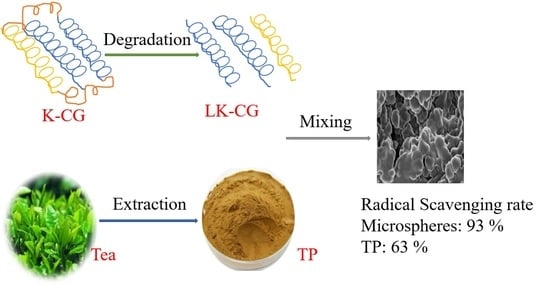
2.2. Preparation of LK-CG
2.3. FTIR Spectroscopy and 13C-NMR of K-CG, LK-CG
2.4. Preparation of LK-CG-TP Microspheres
2.5. Characterization Techniques
2.5.1. SEM Analysis of LK-CG-TP Powder
2.5.2. TG Analysis and DSC Analysis
2.5.3. In Vitro Release Study
2.5.4. DPPH Radical Scavenging Activity of TP and LK-CG-TP
2.6. Statistical Analysis
3. Results
3.1. Preparation and Characterization of LK-CG
3.2. Characterization of LK-CG-TP Microspheres
3.2.1. Morphology of Microspheres
3.2.2. The Thermal Stability of LK-CG-TP Microspheres
3.2.3. Release Profile of EGCG from LK-CG-TP Microsphere
3.2.4. DPPH Scavenging Ratio of LK-CG-TP Microspheres and TP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Bora, A.F.M.; Ma, S.; Li, X.; Liu, L. Application of microencapsulation for the safe delivery of green tea polyphenols in food systems: Review and recent advances. Food Res. Int. 2018, 105, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Bourassa, P.; Kanakis, C.D.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Review on the loading efficacy of dietary tea polyphenols with milk proteins. Food Hydrocoll. 2018, 77, 322–328. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Yang, H.; Yang, X. Tea polyphenols regulate gut microbiota dysbiosis induced by antibiotic in mice. Food Res. Int. 2021, 141, 110153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, R.; Mine, Y. The impact of oolong and black tea polyphenols on human health. Food Biosci. 2019, 29, 55–61. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N. Reactions of plant polyphenols in foods: Impact of molecular structure. Trends Food Sci. Technol. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Shim, S.M.; Yoo, S.H.; Rha, C.S.; Kim, Y.K.; Chung, J.O.; Lee, S.J. Digestive stability and absorption of green tea polyphenols: Influence of acid and xylitol addition. Food Res. Int. 2012, 45, 204–210. [Google Scholar] [CrossRef]
- Li, C.; Li, B.; Zhu, C.; Meng, X. Modeling and optimization of tea polyphenol-alginate/chitosan magnetic microcapsules. J. Mol. Struct. 2020, 1208, 127827. [Google Scholar] [CrossRef]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Varidi, M. Maillard conjugate-based delivery systems for the encapsulation, protection, and controlled release of nutraceuticals and food bioactive ingredients: A review. Food Hydrocoll. 2020, 100, 105389. [Google Scholar] [CrossRef]
- Pasrija, D.; Ezhilarasi, P.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT Food Sci. Technol. 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Peres, I.; Rocha, S.; Gomes, J.; Morais, S.; Pereira, M.D.C.; Coelho, M. Preservation of catechin antioxidant properties loaded in carbohydrate nanoparticles. Carbohydr. Polym. 2011, 86, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Mascaraque, L.G.; Lagarón, J.M.; López-Rubio, A. Electrosprayed gelatin submicroparticles as edible carriers for the encapsulation of polyphenols of interest in functional foods. Food Hydrocoll. 2015, 49, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Otálora, M.C.; Camelo, R.; Wilches-Torres, A.; Cárdenas-Chaparro, A.; Castaño, J.A.G. Encapsulation Effect on the In Vitro Bioaccessibility of Sacha Inchi Oil (Plukenetia volubilis L.) by Soft Capsules Composed of Gelatin and Cactus Mucilage Biopolymers. Polymers 2020, 12, 1995. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Gu, L.; McClements, D.J.; Li, J.; Su, Y.; Yang, Y.; Li, J. Formulation of alginate/carrageenan microgels to encapsulate, protect and release immunoglobulins: Egg Yolk IgY. Food Hydrocoll. 2021, 112, 106349. [Google Scholar] [CrossRef]
- Bakry, A.M.; Huang, J.; Zhai, Y.; Huang, Q. Myofibrillar protein with κ- or λ-carrageenans as novel shell materials for microencapsulation of tuna oil through complex coacervation. Food Hydrocoll. 2019, 96, 43–53. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Xue, C. Recent advances in carrageenan-based delivery systems for bioactive ingredients: A review. Trends Food Sci. Technol. 2021, 112, 348–361. [Google Scholar] [CrossRef]
- He, F.; Kong, Q.; Jin, Z.; Mou, H. Developing a unidirectionally permeable edible film based on k-carrageenan and gelatin for visually detecting the freshness of grass carp fillets. Carbohydr. Polym. 2020, 241, 116336. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Yu, H.; Huang, Q. Encapsulation of epigallocatechin-3-gallate (EGCG) using oil-in-water (O/W) submicrometer emulsions stabilized by iota-carrageenan and beta-lactoglobulin. J. Agric. Food Chem. 2010, 58, 10373–10381. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of kappa-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef]
- Wu, S.J. Degradation of kappa-carrageenan by hydrolysis with commercial alpha-amylase. Carbohydr. Polym. 2012, 89, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Spichtig, V.; Austin, S. Determination of the low molecular weight fraction of food-grade carrageenans. J. Chromatogr. B 2008, 861, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Komes, D.; Karlović, S.; Djaković, S.; Špoljarić, I.; Mršić, G.; Ježek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Tecson, M.G.; Abad, L.V.; Ebajo, V.D., Jr.; Camacho, D.H. Ultrasound-assisted depolymerization of kappa-carrageenan and characterization of degradation product. Ultrason. Sonochem. 2021, 73, 105540. [Google Scholar] [CrossRef]
- Ellis, A.; Jacquier, J.-C. Manufacture of food grade κ-carrageenan microspheres. J. Food Eng. 2009, 94, 316–320. [Google Scholar] [CrossRef]
- Xie, H.; Xiang, C.; Li, Y.; Wang, L.; Zhang, Y.; Song, Z.; Ma, X.; Lu, X.; Lei, Q.; Fang, W. Fabrication of ovalbumin/κ-carrageenan complex nanoparticles as a novel carrier for curcumin delivery. Food Hydrocoll. 2019, 89, 111–121. [Google Scholar] [CrossRef]
- Huang, W.; Wang, L.; Wei, Y.; Cao, M.; Xie, H.; Wu, D. Fabrication of lysozyme/kappa-carrageenan complex nanoparticles as a novel carrier to enhance the stability and in vitro release of curcumin. Int. J. Biol. Macromol. 2020, 146, 444–452. [Google Scholar] [CrossRef]
- Zúñiga, E.A.; Matsuhiro, B.; Mejías, E. Preparation of a low-molecular weight fraction by free radical depolymerization of the sulfated galactan from Schizymenia binderi (Gigartinales, Rhodophyta) and its anticoagulant activity. Carbohydr. Polym. 2006, 66, 208–215. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Z.; Lu, X.; Zeng, S.; Chen, C.; Zhang, L.; Zheng, B. Purification and Characterisation of kappa-Carrageenan Oligosaccharides Prepared by kappa-Carrageenase from Thalassospira sp. Fjfst-332. Carbohydr. Polym. 2018, 180, 314–327. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Jos, B.; Ratnawati, R. Effect of ozonation process on physicochemical and rheological properties of κ-carrageenan. Sci. Study Research. Chem. Chem. Eng. Biotechnol. Food Ind. 2017, 18, 9. [Google Scholar]
- Cosco, D.; Failla, P.; Costa, N.; Pullano, S.; Fiorillo, A.; Mollace, V.; Fresta, M.; Paolino, D. Rutin-loaded chitosan microspheres: Characterization and evaluation of the anti-inflammatory activity. Carbohydr. Polym. 2016, 152, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.W.; Yoo, B. Preparation of milk protein isolate/κ-carrageenan conjugates by maillard reaction in wet-heating system and their application to stabilization of oil-in-water emulsions. LWT 2020, 139, 110542. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Christwardana, M.; Suzery, M.; Sutanto, H.; Nilamsari, A.M.; Yunanda, A. Effects of Carrageenan and Chitosan as Coating Materials on the Thermal Degradation of Microencapsulated Phycocyanin from Spirulina sp. Int. J. Food Eng. 2019, 15, 5–6. [Google Scholar] [CrossRef]
- De Lima Barizao, C.; Crepaldi, M.I.; Oscar de Oliveira, S.; de Oliveira, A.C.; Martins, A.F.; Garcia, P.S.; Bonafe, E.G. Biodegradable films based on commercial kappa-carrageenan and cassava starch to achieve low production costs. Int. J. Biol. Macromol. 2020, 165, 582–590. [Google Scholar] [CrossRef]
- Pourashouri, P.; Shabanpour, B.; Heydari, S.; Raeisi, S. Encapsulation of fish oil by carrageenan and gum tragacanth as wall materials and its application to the enrichment of chicken nuggets. LWT 2021, 137, 110334. [Google Scholar] [CrossRef]
- Ashe, S.; Behera, S.; Dash, P.; Nayak, D.; Nayak, B. Gelatin carrageenan sericin hydrogel composites improves cell viability of cryopreserved SaOS-2 cells. Int. J. Biol. Macromol. 2020, 154, 606–620. [Google Scholar] [CrossRef]
- Tomoda, K.; Asahiyama, M.; Ohtsuki, E.; Nakajima, T.; Terada, H.; Kanebako, M.; Inagi, T.; Makino, K. Preparation and properties of carrageenan microspheres containing allopurinol and local anesthetic agents for the treatment of oral mucositis. Colloids Surf. B Biointerfaces 2009, 71, 27–35. [Google Scholar] [CrossRef]







| Sample | Mw (Da) | Mn (Da) | PDI |
|---|---|---|---|
| K-CG | 233,501.3 | 1,439,993.1 | 16.2165 |
| LK-CG | 13,009.5 | 11,433.8 | 1.13781 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Wu, K.; Xu, J.; Hu, Z.; Zhang, X. Low Molecular Weight Kappa-Carrageenan Based Microspheres for Enhancing Stability and Bioavailability of Tea Polyphenols. Processes 2021, 9, 1240. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9071240
Feng T, Wu K, Xu J, Hu Z, Zhang X. Low Molecular Weight Kappa-Carrageenan Based Microspheres for Enhancing Stability and Bioavailability of Tea Polyphenols. Processes. 2021; 9(7):1240. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9071240
Chicago/Turabian StyleFeng, Tao, Kai Wu, Jianying Xu, Zhongshan Hu, and Xiaolei Zhang. 2021. "Low Molecular Weight Kappa-Carrageenan Based Microspheres for Enhancing Stability and Bioavailability of Tea Polyphenols" Processes 9, no. 7: 1240. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9071240