Distribution of Sulfate-Reducing Bacteria in the Environment: Cryopreservation Techniques and Their Potential Storage Application
Abstract
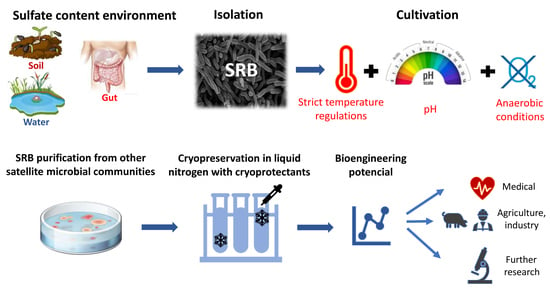
:1. Introduction
2. Sulfate-Reducing Bacteria in Various Biotopes
- Gram-negative mesophilic SRB; these do not form spores and are one of the most widespread SRB in nature (genera Desulfovibrio, Desulfobotulus, Desulfobulbus, Desulfohalobium and Desulfomicrobium);
- Gram-positive spore-forming bacteria; these are a typical representative (genus Desulfotomaculum) that can be identified from soil samples (according to the updated classification, these microorganisms are represented and included in order Clostridiales);
- Gram-negative thermophilic sulfate-reducing microorganisms (genus Thermodesulfobacterium);
- Gram-negative thermophilic archaeal sulfate-reducing microorganisms; these include members of the genus Archaeoglobus that can only be found in anaerobic, underwater, hydrothermal environments because they require salt and high temperature for their growth.
2.1. Water Environment
2.2. Surfaces of Corrosive Metals
2.3. Corrosion of Concrete, Stone Elements and Masonry
2.4. Gastrointestinal Tract
3. Conditions Determining the Viability of Sulfate-Reducing Bacteria
3.1. Physical Conditions
3.2. Competition and Coexistence with Other Intestinal Microorganisms
3.3. Biochemical Characteristics of Sulfate-Reducing Bacteria
4. Cultivation and Storage of Sulfate-Reducing Bacteria
4.1. Isolation of Intestinal Sulfate-Reducing Bacteria
- The entire tube or Eppendorf is filled to the brim with Postgate medium;
- One mL of sterile liquid paraffin is added dropwise to the surface of the medium, thus keeping anaerobic conditions.
4.2. Media for Cultivation of Sulfate-Reducing Bacteria
5. Preservation of Intestinal Microorganisms
5.1. Cryopreservation of Intestinal Microorganisms
5.2. Methods of Cryopreservation
5.3. Laboratory Equipment, Instruments and Materials
5.4. Cryoprotectants
5.5. Viability
5.5.1. Stress Factors
- Cooling to temperatures above 0 °C (cold shock);
- Further gradual cooling below 0 °C (frost damage);
- Storage temperature;
- Heating to room temperature (defrost damage);
- Recovery.
5.5.2. Osmoregulation and Osmotic Stress
6. Summary of the Obtained Information with the Possibility of Application to SRB
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cambridge University Press. Sulphate-Reducing Bacteria: Environmental and Engineered Systems; Barton, L., Hamilton, W.A., Eds.; Cambridge University Press: Cambridge, NY, USA, 2007; ISBN 978-0-521-85485-6. [Google Scholar]
- Barton, L.L.; Fauque, G.D. Chapter 2 Biochemistry, Physiology and Biotechnology of Sulfate-Reducing Bacteria. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 68, pp. 41–98. ISBN 978-0-12-374803-4. [Google Scholar]
- Kushkevych, I. Isolation and Purification of Sulfate-Reducing Bacteria. In Microorganisms; Blumenberg, M., Shaaban, M., Elgaml, A., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-83880-187-8. [Google Scholar]
- Kushkevych, I.; Dordević, D.; Kollár, P. Analysis of Physiological Parameters of Desulfovibrio Strains from Individuals with Colitis. Open Life Sci. 2019, 13, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Analysis of PH Dose-Dependent Growth of Sulfate-Reducing Bacteria. Open Med. 2019, 14, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Kollar, P.; Suchy, P.; Parak, T.; Pauk, K.; Imramovsky, A. Activity of Selected Salicylamides against Intestinal Sulfate-Reducing Bacteria. Neuro Endocrinol. Lett. 2015, 36 (Suppl. 1), 106–113. [Google Scholar]
- Kushkevych, I.V. Kinetic Properties of Pyruvate Ferredoxin Oxidoreductase of Intestinal Sulfate-Reducing Bacteria Desulfovibrio Piger Vib-7 and Desulfomicrobium Sp. Rod-9. Pol. J. Microbiol. 2015, 64, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Fafula, R.; Parák, T.; Bartoš, M. Activity of Na+/K+-Activated Mg2+-Dependent ATP-Hydrolase in the Cell-Free Extracts of the Sulfate-Reducing Bacteria Desulfovibrio Piger Vib-7 and Desulfomicrobium Sp. Rod-9. Acta Vet. Brno 2015, 84, 3–12. [Google Scholar] [CrossRef]
- Kushkevych, I.V. Activity and Kinetic Properties of Phosphotransacetylase from Intestinal Sulfate-Reducing Bacteria. Acta Biochim. Pol. 2015, 62, 103–108. [Google Scholar] [CrossRef]
- Kushkevych, I.; Coufalová, M.; Vítězová, M.; Rittmann, S.K.-M.R. Sulfate-Reducing Bacteria of the Oral Cavity and Their Relation with Periodontitis—Recent Advances. JCM 2020, 9, 2347. [Google Scholar] [CrossRef]
- Ran, S.; Mu, C.; Zhu, W. Diversity and Community Pattern of Sulfate-Reducing Bacteria in Piglet Gut. J. Animal Sci. Biotechnol. 2019, 10, 40. [Google Scholar] [CrossRef]
- Postgate, J. The Suphate-Reducing Bacteria, 2nd ed.; Cambridge University: Cambridge, NY, USA, 1984. [Google Scholar]
- Hubálek, Z. Cryopreservation of Microorganisms at Ultra-Low Temperatures; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Trsic-Milanovic, N.; Kodzic, A.; Baras, J.; Dimitrijevic-Brankovic, S. The Influence of a Cryoprotective Medium Containing Glycerol on the Lyophilization of Lactic Acid Bacteria. J. Serb. Chem. Soc. 2001, 66, 435–441. [Google Scholar] [CrossRef]
- Butterfield, W.; Jong, S.C.; Alexander, M.T. Polypropylene Vials for Preserving Fungi in Liquid Nitrogen. Mycologia 1978, 70, 1122–1124. [Google Scholar] [CrossRef]
- Grout, B.; Morris, J.; Mclellan, M. Cryopreservation and the Maintenance of Cell Lines. Trends Biotechnol. 1990, 8, 293–297. [Google Scholar] [CrossRef]
- Castro, H.F.; Williams, N.H.; Ogram, A. Phylogeny of Sulfate-Reducing Bacteria1. FEMS Microbiol. Ecol. 2000, 31, 1–9. [Google Scholar] [CrossRef]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T.; Garrity, G.M. The Proteobacteria, Part C: The Alpha-, Beta-, Delta-, and Epsilonproteobacteria. In Bergey’s Manual of Systematic Bacteriology; Springer: Boston, MA, USA, 2005; p. 1388. [Google Scholar]
- Kushkevych, I.; Vítězová, M.; Kos, J.; Kollár, P.; Jampílek, J. Effect of Selected 8-Hydroxyquinoline-2-Carboxanilides on Viability and Sulfate Metabolism of Desulfovibrio Piger. J. Appl. Biomed. 2018, 16, 241–246. [Google Scholar] [CrossRef]
- Kushkevych, I.; Cejnar, J.; Treml, J.; Dordević, D.; Kollar, P.; Vítězová, M. Recent Advances in Metabolic Pathways of Sulfate Reduction in Intestinal Bacteria. Cells 2020, 9, 698. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Kováč, J.; Vítězová, M.; Vítěz, T.; Bartoš, M. The Diversity of Sulfate-Reducing Bacteria in the Seven Bioreactors. Arch. Microbiol. 2018, 200, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Abdulina, D.; Kováč, J.; Iutynska, G.; Kushkevych, I. ATP Sulfurylase Activity of Sulfate-Reducing Bacteria from Various Ecotopes. Biotech 2020, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Fauque, G.D. Ecology of Sulfate-Reducing Bacteria. In Sulfate-Reducing Bacteria; Barton, L.L., Ed.; Springer: Boston, MA, USA, 1995; pp. 217–241. ISBN 978-1-4899-1584-9. [Google Scholar]
- Hao, O.J.; Chen, J.M.; Huang, L.; Buglass, R.L. Sulfate-reducing Bacteria. Crit. Rev. Environ. Sci. Technol. 1996, 26, 155–187. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen Sulfide Toxicity in the Gut Environment: Meta-Analysis of Sulfate-Reducing and Lactic Acid Bacteria in Inflammatory Processes. J. Adv. Res. 2020, 27, 55–69. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Possible Synergy Effect of Hydrogen Sulfide and Acetate Produced by Sulfate-Reducing Bacteria on Inflammatory Bowel Disease Development. J. Adv. Res. 2020, 21, 71–78. [Google Scholar] [CrossRef]
- Černý, M.; Vítězová, M.; Vítěz, T.; Bartoš, M.; Kushkevych, I. Variation in the Distribution of Hydrogen Producers from the Clostridiales Order in Biogas Reactors Depending on Different Input Substrates. Energies 2018, 11, 3270. [Google Scholar] [CrossRef] [Green Version]
- Kováč, J.; Vítězová, M.; Kushkevych, I. Metabolic Activity of Sulfate-Reducing Bacteria from Rodents with Colitis. Open Med. 2018, 13, 344–349. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Fedrová, P.; Vochyanová, Z.; Paráková, L.; Hošek, J. Kinetic Properties of Growth of Intestinal Sulphate-Reducing Bacteria Isolated from Healthy Mice and Mice with Ulcerative Colitis. Acta Vet. Brno 2017, 86, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Plugge, C.M.; Zhang, W.; Scholten, J.C.M.; Stams, A.J.M. Metabolic Flexibility of Sulfate-Reducing Bacteria. Front. Microbio. 2011, 2, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köpke, B.; Wilms, R.; Engelen, B.; Cypionka, H.; Sass, H. Microbial Diversity in Coastal Subsurface Sediments: A Cultivation Approach Using Various Electron Acceptors and Substrate Gradients. AEM 2005, 71, 7819–7830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, S.; Itoh, T.; Satoh, H.; Watanabe, Y. Analyses of Spatial Distributions of Sulfate-Reducing Bacteria and Their Activity in Aerobic Wastewater Biofilms. Appl. Environ. Microbiol. 1999, 65, 5107–5116. [Google Scholar] [CrossRef] [Green Version]
- Newport, P.J.; Nedwell, D.B. The Mechanisms of Inhibition of Desulfovibrio and Desulfotomaculum Species by Selenate and Molybdate. J. Appl. Bacteriol. 1988, 65, 419–423. [Google Scholar] [CrossRef]
- Islander, R.L.; Devinny, J.S.; Mansfeld, F.; Postyn, A.; Shih, H. Microbial Ecology of Crown Corrosion in Sewers. J. Environ. Eng. 1991, 117, 751–770. [Google Scholar] [CrossRef]
- Leclerc, H.; Oger, C.; Beerens, H.; Mossel, D.A.A. Occurrence of Sulphate Reducing Bacteria in the Human Intestinal Flora and in the Aquatic Environment. Water Res. 1980, 14, 253–256. [Google Scholar] [CrossRef]
- Gibson, G.R.; Macfarlane, G.T.; Cummings, J.H. Occurrence of Sulphate-Reducing Bacteria in Human Faeces and the Relationship of Dissimilatory Sulphate Reduction to Methanogenesis in the Large Gut. J. Appl. Bacteriol. 1988, 65, 103–111. [Google Scholar] [CrossRef]
- Gibson, G.R.; Macfarlane, S.; Macfarlane, G.T. Metabolic Interactions Involving Sulphate-Reducing and Methanogenic Bacteria in the Human Large Intestine. FEMS Microbiol. Ecol. 1993, 12, 117–125. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Vítěz, T.; Bartoš, M. Production of Biogas: Relationship between Methanogenic and Sulfate-Reducing Microorganisms. Open Life Sci. 2017, 12, 82–91. [Google Scholar] [CrossRef]
- Kushkevych, I.; Vítězová, M.; Vítěz, T.; Kováč, J.; Kaucká, P.; Jesionek, W.; Bartoš, M.; Barton, L. A New Combination of Substrates: Biogas Production and Diversity of the Methanogenic Microorganisms. Open Life Sci. 2018, 13, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Spengler, G.; Urbán, E. Identification and Antimicrobial Susceptibility Testing of Anaerobic Bacteria: Rubik’s Cube of Clinical Microbiology? Antibiotics 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajdács, M.; Urbán, E. Relevance of Anaerobic Bacteremia in Adult Patients: A Never-Ending Story? Eur. J. Microbiol. Immunol. 2020, 10, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Ábrók, M.; Lázár, A.; Terhes, G.; Urbán, E. Anaerobic Blood Culture Positivity at a University Hospital in Hungary: A 5-Year Comparative Retrospective Study. Anaerobe 2020, 63, 102200. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Macfarlane, G.T. Chemostat Enrichment of Sulphate-Reducing Bacteria from the Large Gut. Lett. Appl. Microbiol. 1988, 7, 127–133. [Google Scholar] [CrossRef]
- Gibson, G.R.; Cummings, J.H.; Macfarlane, G.T. Growth and Activities of Sulphate-Reducing Bacteria in Gut Contents of Healthy Subjects and Patients with Ulcerative Colitis. FEMS Microbiol. Lett. 1991, 86, 103–112. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Kollar, P.; Vítězová, M.; Drago, L. Hydrogen Sulfide as a Toxic Product in the Small-Large Intestine Axis and Its Role in IBD Development. JCM 2019, 8, 1054. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Kotrsová, V.; Dordević, D.; Buňková, L.; Vítězová, M.; Amedei, A. Hydrogen Sulfide Effects on the Survival of Lactobacilli with Emphasis on the Development of Inflammatory Bowel Diseases. Biomolecules 2019, 9, 752. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Castro Sangrador, J.; Dordević, D.; Rozehnalová, M.; Černý, M.; Fafula, R.; Vítězová, M.; Rittmann, S.K.-M.R. Evaluation of Physiological Parameters of Intestinal Sulfate-Reducing Bacteria Isolated from Patients Suffering from IBD and Healthy People. JCM 2020, 9, 1920. [Google Scholar] [CrossRef] [PubMed]
- Florin, T.H.J.; Neale, G.; Goretski, S.; Cummings, J.H. The Sulfate Content of Foods and Beverages. J. Food Compos. Anal. 1993, 6, 140–151. [Google Scholar] [CrossRef]
- Kováč, J.; Kushkevych, I. New Modification of Cultivation Medium for Isolation and Growth of Intestinal Sulfate-Reducing Bacteria. In Proceedings of the 24th International Ph.D. Students Conference, Brno, Czech Republic, 8–9 November 2017; Volume 2017, pp. 702–707. [Google Scholar]
- Fallingborg, J.; Christensen, L.A.; Ingeman-Nielsen, M.; Jacobsen, B.A.; Abildgaard, K.; Rasmussen, H.H. PH-Profile and Regional Transit Times of the Normal Gut Measured by a Radiotelemetry Device. Aliment. Pharmacol. Ther. 2007, 3, 605–614. [Google Scholar] [CrossRef]
- Beerens, H.; Romond, C. Sulfate-Reducing Anaerobic Bacteria in Human Feces. Am. J. Clin. Nutr. 1977, 30, 1770–1776. [Google Scholar] [CrossRef] [Green Version]
- Fite, A. Identification and Quantitation of Mucosal and Faecal Desulfovibrios Using Real Time Polymerase Chain Reaction. Gut 2004, 53, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Hilton, B.L.; Oleszkiewicz, J.A. Sulfide-Induced Inhibition of Anaerobic Digestion. J. Environ. Eng. 1988, 114, 1377–1391. [Google Scholar] [CrossRef]
- Christl, S.U.; Gibson, G.R.; Cummings, J.H. Role of Dietary Sulphate in the Regulation of Methanogenesis in the Human Large Intestine. Gut 1992, 33, 1234–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deplancke, B.; Hristova, K.R.; Oakley, H.A.; McCracken, V.J.; Aminov, R.; Mackie, R.I.; Gaskins, H.R. Molecular Ecological Analysis of the Succession and Diversity of Sulfate-Reducing Bacteria in the Mouse Gastrointestinal Tract. Appl. Environ. Microbiol. 2000, 66, 2166–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushkevych, I.; Dordević, D.; Vítězová, M.; Kollár, P. Cross-Correlation Analysis of the Desulfovibrio Growth Parameters of Intestinal Species Isolated from People with Colitis. Biologia 2018, 73, 1137–1143. [Google Scholar] [CrossRef]
- Postgate, J.R. On the Nutrition of Desulphovibrio Desulphuricans. J. Gen. Microbiol. 1951, 5, 714–724. [Google Scholar] [CrossRef] [Green Version]
- Postgate, J.R. Sulphate Reduction by Bacteria. Annu. Rev. Microbiol. 1959, 13, 505–520. [Google Scholar] [CrossRef]
- Postgate, J.R.; Campbell, L.L. Classification of Desulfovibrio Species, the Nonsporulating Sulfate-Reducing Bacteria. Bacteriol. Rev. 1966, 30, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Bade, K.; Manz, W.; Szewzyk, U. Behavior of Sulfate Reducing Bacteria under Oligotrophic Conditions and Oxygen Stress in Particle-Free Systems Related to Drinking Water. FEMS Microbiol. Ecol. 2000, 32, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Krekeler, D.; Sigalevich, P.; Teske, A.; Cypionka, H.; Cohen, Y. A Sulfate-Reducing Bacterium from the Oxic Layer of a Microbial Mat from Solar Lake (Sinai), Desulfovibrio oxyclinae Sp. Nov. Arch. Microbiol. 1997, 167, 369–375. [Google Scholar] [CrossRef]
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. Evidence That Hydrogen Sulfide Is a Genotoxic Agent. Mol. Cancer Res. 2006, 4, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushkevych, I.; Leščanová, O.; Dordević, D.; Jančíková, S.; Hošek, J.; Vítězová, M.; Buňková, L.; Drago, L. The Sulfate-Reducing Microbial Communities and Meta-Analysis of Their Occurrence during Diseases of Small–Large Intestine Axis. JCM 2019, 8, 1656. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Dordević, D.; Vítězová, M. Toxicity of Hydrogen Sulfide toward Sulfate-Reducing Bacteria Desulfovibrio Piger Vib-7. Arch. Microbiol. 2019, 201, 389–397. [Google Scholar] [CrossRef]
- Pitcher, M.C.; Cummings, J.H. Hydrogen Sulphide: A Bacterial Toxin in Ulcerative Colitis? Gut 1996, 39, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bircher, L.; Geirnaert, A.; Hammes, F.; Lacroix, C.; Schwab, C. Effect of Cryopreservation and Lyophilization on Viability and Growth of Strict Anaerobic Human Gut Microbes. Microb. Biotechnol. 2018, 11, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, D.V.; Zalomova, L.V.; Zagainova, A.V.; Makarov, V.V.; Mezhevikina, L.M.; Fesenko, E.E.; Yudin, S.M. Cryopreservation of the Human Gut Microbiota: Current State and Perspectives. Int. J. Med. Microbiol. 2019, 309, 259–269. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.E.; Galley, J.D.; Hufnagle, A.R.; Allen, R.G.; Lyte, M. Exposure to a Social Stressor Alters the Structure of the Intestinal Microbiota: Implications for Stressor-Induced Immunomodulation. Brain Behav. Immun. 2011, 25, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Blaser, M.J.; Falkow, S. What Are the Consequences of the Disappearing Human Microbiota? Nat. Rev. Microbiol. 2009, 7, 887–894. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-Term Antibiotic Treatment Has Differing Long-Term Impacts on the Human Throat and Gut Microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giulio, B.D.; Orlando, P.; Barba, G.; Coppola, R.; Rosa, M.D.; Sada, A.; Prisco, P.P.D.; Nazzaro, F. Use of Alginate and Cryo-Protective Sugars to Improve the Viability of Lactic Acid Bacteria after Freezing and Freeze-Drying. World J. Microbiol. Biotechnol. 2005, 21, 739–746. [Google Scholar] [CrossRef]
- Perry, S.F. Freeze-Drying and Cryopreservation of Bacteria. Mol. Biotechnol. 1998, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.; Marechal, P.-A.; Gervais, P. Cell Size and Water Permeability as Determining Factors for Cell Viability after Freezing at Different Cooling Rates. Appl. Environ. Microbiol. 2004, 70, 268–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nova Biomedical. Cryopreservation: Technologies, Applications, and Risks/Outcomes; Colvert, A., Coty, H., Eds.; Cell biology research progress; Nova Biomedical: New York, NY, USA, 2013; ISBN 978-1-62618-475-6. [Google Scholar]
- Calcott, P.H.; MacLeod, R.A. Survival of Escherichia Coli from Freeze–Thaw Damage: A Theoretical and Practical Study. Can. J. Microbiol. 1974, 20, 671–681. [Google Scholar] [CrossRef]
- Calcott, P.H.; MacLeod, R.A. Survival of Escherichia Coli from Freeze-Thaw Damage: Influence of Nutritional Status and Growth Rate. Can. J. Microbiol. 1974, 20, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, W.; Jong, S.C.; Alexander, M.T. Preservation of Living Fungi Pathogenic for Man and Animals. Can. J. Microbiol. 1974, 20, 1665–1673. [Google Scholar] [CrossRef]
- Shannon, J.E.; Macy, M.L. Freezing, Storage, and Recovery of Cell Stocks. In Tissue Culture; Elsevier: Amsterdam, The Netherlands, 1973; pp. 712–718. ISBN 978-0-12-427150-0. [Google Scholar]
- Malik, K.A. A New Method for Liquid Nitrogen Storage of Phototrophic Bacteria under Anaerobic Conditions. J. Microbiol. Methods 1984, 2, 41–47. [Google Scholar] [CrossRef]
- Malik, K.A. Maintenance of phototrophic bacteria. In Maintenance of Microorganisms and Cultured Cells; The Department for Environment, Food and Rural Affairs: London, UK, 1991. [Google Scholar]
- Hofmann, P. Cryopreservation of Fungi. World J. Microbiol. Biotechnol. 1991, 7, 92–94. [Google Scholar] [CrossRef]
- Jackson, P.R.; Jackson, J.E.; Raney, D.E. A Sterile Leakproof Plastic Vial for Cell Cryopreservation in Liquid Nitrogen: Application to Parasitic Protozoa. Cryobiology 1981, 18, 608–611. [Google Scholar] [CrossRef]
- Simione, F.P.; Daggett, P.-M. Recovery of a Marine Dinoflagellate Following Controlled and Uncontrolled Freezing. Cryobiology 1977, 14, 362–366. [Google Scholar] [CrossRef]
- Simione, F.P.; Daggett, P.-M.; McGrath, M.S.; Alexander, M.T. The Use of Plastic Ampoules for Freeze Preservation of Microorganisms. Cryobiology 1977, 14, 500–502. [Google Scholar] [CrossRef]
- Hubálek, Z. Protectants Used in the Cryopreservation of Microorganisms. Cryobiology 2003, 46, 205–229. [Google Scholar] [CrossRef]
- Heylen, K.; Hoefman, S.; Vekeman, B.; Peiren, J.; De Vos, P. Safeguarding Bacterial Resources Promotes Biotechnological Innovation. Appl. Microbiol. Biotechnol. 2012, 94, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Taubner, R.-S.; Schleper, C.; Firneis, M.; Rittmann, S. Assessing the Ecophysiology of Methanogens in the Context of Recent Astrobiological and Planetological Studies. Life 2015, 5, 1652–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, K.; Hayashi, F.; Nagashima, T.; Rajan, R.; Hyon, S.-H. Molecular Mechanisms of Cell Cryopreservation with Polyampholytes Studied by Solid-State NMR. Commun. Mater. 2021, 2, 15. [Google Scholar] [CrossRef]
- Fonseca, F.; Béal, C.; Corrieu, G. Operating Conditions That Affect the Resistance of Lactic Acid Bacteria to Freezing and Frozen Storage. Cryobiology 2001, 43, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Guérin-Danan, C. Storage of Intestinal Bacteria in Samples Frozen with Glycerol. Microb. Ecol. Health Dis. 1999, 11, 180–182. [Google Scholar] [CrossRef]
- Calcott, P.H.; MacLeod, R.A. The survival of Escherichia coli from freeze–thaw damage: The relative importance of wall and membrane damage. Can. J. Microbiol. 1975, 21, 1960–1968. [Google Scholar] [CrossRef]
- Fowler, A. Cryo-Injury and Biopreservation. Ann. N. Y. Acad. Sci. 2005, 1066, 119–135. [Google Scholar] [CrossRef]
- Smittle, R.B.; Gilliland, S.E.; Speck, M.L. Death of Lactobacillus Bulgaricus Resulting from Liquid Nitrogen Freezing. Appl. Microbiol. 1972, 24, 551–554. [Google Scholar] [CrossRef]
- Ruwart, M.J.; Holland, J.F.; Haug, A. Fluorimetric Evidence of Interactions Involving Cryoprotectants and Biomolecules. Cryobiology 1975, 12, 26–33. [Google Scholar] [CrossRef]
- Heckly, R.J. Preservation of Microorganisms. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 24, pp. 1–53. ISBN 978-0-12-002624-1. [Google Scholar]
- Streit, F.; Delettre, J.; Corrieu, G.; Béal, C. Acid Adaptation of Lactobacillus Delbrueckii Subsp. Bulgaricus Induces Physiological Responses at Membrane and Cytosolic Levels That Improves Cryotolerance. J. Appl. Microbiol. 2008, 105, 1071–1080. [Google Scholar] [CrossRef]
- Williams, W.P.; Chapman, D.; Heber, U.; Franks, F.; Jaenicke, R. Cold-Induced Lipid Phase Transitions [and Discussion]. In Philosophical Transactions of the Royal Society of London B: Biological Sciences; The Royal Society Publishing: London, UK, 1990; Volume 326, pp. 555–570. [Google Scholar]
- Péter, G.; Reichart, O. The Effect of Growth Phase, Cryoprotectants and Freezing Rates on the Survival of Selected Microorganisms during Freezing and Thawing. Acta Aliment. 2001, 30, 89–97. [Google Scholar] [CrossRef]
- Tanno, K. An Explanation for the Cell Concentration Dependent Survival of Escherichia Coli after Freeze-Thawing. Ser. B Biol. Sci. 1979, 57–59. [Google Scholar]
- Gao, D.; Critser, J.K. Mechanisms of Cryoinjury in Living Cells. ILAR J. 2000, 41, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur, P.; Leibo, S.P.; Chu, E.H.Y. A Two-Factor Hypothesis of Freezing Injury. Exp. Cell Res. 1972, 71, 345–355. [Google Scholar] [CrossRef]
- Mazur, P. The Role of Intracellular Freezing in the Death of Cells Cooled at Supraoptimal Rates. Cryobiology 1977, 14, 251–272. [Google Scholar] [CrossRef]
- Mishra, S.; Imlay, J.A. An Anaerobic Bacterium, Bacteroides Thetaiotaomicron, Uses a Consortium of Enzymes to Scavenge Hydrogen Peroxide: H2O2 Scavenging Enzymes in Bacteroides Thetaiotaomicron. Mol. Microbiol. 2013, 90, 1356–1371. [Google Scholar] [CrossRef] [Green Version]
- Pan, N.; Imlay, J.A. How Does Oxygen Inhibit Central Metabolism in the Obligate Anaerobe Bacteroides Thetaiotaomicron. Mol. Microbiol. 2001, 39, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; van Dijl, J.M.; Harmsen, H.J.M. Antioxidants Keep the Potentially Probiotic but Highly Oxygen-Sensitive Human Gut Bacterium Faecalibacterium Prausnitzii Alive at Ambient Air. PLoS ONE 2014, 9, e96097. [Google Scholar] [CrossRef]
- Cebrián, G.; Arroyo, C.; Mañas, P.; Condón, S. Bacterial Maximum Non-Inhibitory and Minimum Inhibitory Concentrations of Different Water Activity Depressing Solutes. Int. J. Food Microbiol. 2014, 188, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Saegeman, V.S.M.; Ectors, N.L.; Lismont, D.; Verduyckt, B.; Verhaegen, J. Short- and Long-Term Bacterial Inhibiting Effect of High Concentrations of Glycerol Used in the Preservation of Skin Allografts. Burns 2008, 34, 205–211. [Google Scholar] [CrossRef]
- Kuever, J. The Family Desulfovibrionaceae. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 107–133. ISBN 978-3-642-39043-2. [Google Scholar]
- van der Maarel, M.J.E.C.; van Bergeijk, S.; van Werkhoven, A.F.; Laverman, A.M.; Meijer, W.G.; Stam, W.T.; Hansen, T.A. Cleavage of Dimethylsulfoniopropionate and Reduction of Acrylate by Desulfovibrio Acrylicus Sp. Nov. Arch. Microbiol. 1996, 166, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Kushkevych, I.; Abdulina, D.; Kováč, J.; Dordević, D.; Vítězová, M.; Iutynska, G.; Rittmann, S.K.-M.R. Adenosine-5′-Phosphosulfate- and Sulfite Reductases Activities of Sulfate-Reducing Bacteria from Various Environments. Biomolecules 2020, 10, 921. [Google Scholar] [CrossRef]






| Salts (g L−1) | Postgate B | Postgate C | Postgate E | Modified by Kovac & Kushkevych, 2017 [49] |
|---|---|---|---|---|
| Na2SO4 | – | 4.5 | 1 | 3 |
| KH2PO4 | 0.5 | 0.5 | 0.5 | 0.3 |
| K2HPO4 | – | – | – | 0.5 |
| NH4Cl | 1 | 1 | 1 | 1 |
| CaCl2·6H20 | – | 0.06 | 1 | 0.06 |
| Yeast extract | 1 | 1 | 1 | 1 |
| MgSO4·7H2O | 2 | 0.06 | 2 | 0.1 |
| CaSO4 | 1 | – | – | – |
| Ascorbic acid | 0.1 | – | 0.1 | 0.1 |
| Thioglycolic acid | 0.1 | – | – | – |
| (NH4)2SO4 | – | – | – | 0.2 |
| Lactobacillus bulgaricus | Medium | Death (%) | Loss of Acid Production (%) |
|---|---|---|---|
| L. bulgaricus NCS1 | –T80 | 67 | 5 |
| +T80 | 3 | 1 | |
| L. bulgaricus NCS2 | –T80 | 83 | 32 |
| +T80 | 38 | 9 | |
| L. bulgaricus NCS3 | –T80 | 65 | 70 |
| +T80 | 49 | 40 | |
| L. bulgaricus NCS4 | –T80 | 7 | 7 |
| +T80 | 0 | 5 |
| Lactobacillus bulgaricus | Storage Time | |||
|---|---|---|---|---|
| 1. Day | 2. Day | |||
| Death (%) | Loss of Acid Production (%) | Death (%) | Loss of Acid Production (%) | |
| L. bulgaricus NCS1 | 95 | 73 | 99 | 69 |
| L. bulgaricus NCS3 | 54 | 31 | 72 | 32 |
| L. bulgaricus NCS4 | 0 | 8 | 0 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushkevych, I.; Kovářová, A.; Dordevic, D.; Gaine, J.; Kollar, P.; Vítězová, M.; Rittmann, S.K.-M.R. Distribution of Sulfate-Reducing Bacteria in the Environment: Cryopreservation Techniques and Their Potential Storage Application. Processes 2021, 9, 1843. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9101843
Kushkevych I, Kovářová A, Dordevic D, Gaine J, Kollar P, Vítězová M, Rittmann SK-MR. Distribution of Sulfate-Reducing Bacteria in the Environment: Cryopreservation Techniques and Their Potential Storage Application. Processes. 2021; 9(10):1843. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9101843
Chicago/Turabian StyleKushkevych, Ivan, Aneta Kovářová, Dani Dordevic, Jonah Gaine, Peter Kollar, Monika Vítězová, and Simon K.-M. R. Rittmann. 2021. "Distribution of Sulfate-Reducing Bacteria in the Environment: Cryopreservation Techniques and Their Potential Storage Application" Processes 9, no. 10: 1843. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9101843