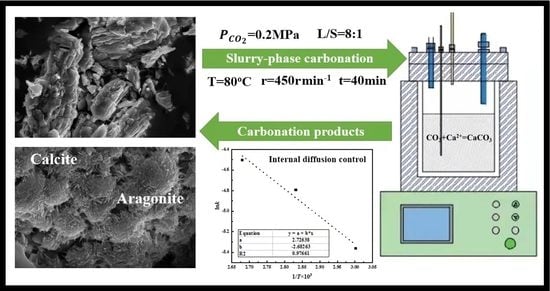
Slurry-Phase Carbonation Reaction Characteristics of AOD Stainless Steel Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Original AOD Slag
2.1.1. Chemical Composition of AOD Slag
2.1.2. Mineral Composition of AOD Slag
2.2. Carbonation Experiment of AOD Slag
2.2.1. Slurry-Phase Accelerated Carbonation
2.2.2. Carbonation Process
2.3. Thermogravimetric Experiment
2.4. Microscopic Morphology Analysis
3. Results and Discussion
3.1. Effect of Stirring Speed on the Carbonation of AOD Slag
3.2. The Effect of Reaction Temperature on Carbonation of AOD Slag
3.3. Carbonation Products of AOD Slag
3.3.1. XRD Analysis
3.3.2. FTIR Analysis
3.3.3. Microscopic Morphology Analysis
3.4. Carbonation Reaction Kinetic Model of AOD Slag
4. Conclusions
- (1)
- A higher stirring speed could accelerate the reaction rate in the early stage (0–10 min) of the slurry-phase carbonation reaction, but when the carbonation reaction entered the later stage and reached the maximum carbonation ratio, the stirring speed did not improve the carbonation potential of the AOD slag.
- (2)
- The slurry-phase carbonation of AOD slag had a relatively violent initial reaction, and the maximum carbonation ratio could be reached when the reaction reached 20–40 min. In the lower temperature range (60–80 °C), as the reaction temperature increased, the number of activated carbonation molecules in the slurry-phase carbonation system per unit time increased, which increased the carbonation efficiency of AOD slag. In the higher temperature range (80 °C–100 °C), the activation efficiency of the reaction temperature on the carbonation activity of AOD slag decreased.
- (3)
- The carbonation products of the AOD slag were mainly calcium carbonates with different crystal forms. As the reaction temperature increased, the mass fraction of calcite (CaCO3(C)) in the carbonated AOD slag gradually increased. When the reaction temperature was 60 °C, the crystal form was single calcite (CaCO3(C)), while when the reaction temperature was 80 °C or 100 °C, aragonite (CaCO3(A)) began to precipitate in the carbonated AOD slag.
- (4)
- Within 20 min from the beginning to the equilibrium of the reaction, the slurry-phase carbonation of AOD slag was controlled by shell diffusion (internal diffusion), and its activation energy was 22.28 kJ/mol.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.Q.; Qi, Y.H.; Shi, Y.L.; Na, X.Z.; Feng, H.L. Mechanism and prevention of disintegration of AOD stainless steel slag. J. Iron Steel Res. Int. 2013, 20, 26–30. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.G.; Wang, Y.J.; Zeng, Y.N.; Gao, A.M. Properties of aged AOD slag and its leaching characteristics. China Metall. 2021, 31, 104–110. [Google Scholar]
- Adegoloye, G.; Beaucour, A.L.; Ortola, S.; Noumowe, A. Concretes made of EAF slag and AOD slag aggregates from stainless steel process: Mechanical properties and durability. Constr. Build. Mater. 2015, 76, 313–321. [Google Scholar] [CrossRef]
- Zhang, H.; Xin, H. An overview for the utilization of wastes from stainless steel industries. Resour. Conserv. Recycl. 2011, 55, 745–754. [Google Scholar]
- Liu, B.; Li, J.; Zeng, Y.; Wang, Z. Toxicity assessment and geochemical model of chromium leaching from AOD slag. Chemosphere 2016, 144, 2052–2057. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, W.; Gao, F.; Huang, H.; Zhao, L. An overview of resource utilization of steel slag as absorbent material for waste water treatment. E3S Web Conf. 2020, 199, 17. [Google Scholar] [CrossRef]
- Yadav, S.; Mehra, A. Dissolution of steel slags in aqueous media. Environ. Sci. Pollut. Res. 2017, 24, 16305–16315. [Google Scholar]
- Yadav, S.; Mehra, A. Experimental study of dissolution of minerals and CO2 sequestration in steel slag. Waste Manag. 2017, 64, 348–357. [Google Scholar] [CrossRef]
- Ghouleh, Z.; Guthrie, R.; Shao, Y. Production of carbonate aggregates using steel slag and carbon dioxide for carbon-negative concrete. J. CO2 Util. 2017, 18, 125–138. [Google Scholar] [CrossRef]
- Santos, R.M.; Bouwel, J.V.; Vandevelde, E.; Mertens, G.; Elsen, J.; Gerven, T.V. Accelerated mineral carbonation of stainless steel slags for CO2 storage and waste valorization: Effect of process parameters on geochemical properties. Int. J. Greenh. Gas Control. 2013, 17, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Saran, R.K.; Arora, V.; Yadav, S. CO2 Sequestration by Mineral Carbonation: A Review. Glob. Nest. J. 2018, 20, 497–503. [Google Scholar]
- Chang, E.E.; Chen, C.H.; Chen, Y.H.; Pan, S.Y.; Chiang, P.C. Performance evaluation for carbonation of steel-making slags in a slurry reactor. J. Hazard. Mater. 2011, 186, 558–564. [Google Scholar]
- Salman, M.; Cizer, O.; Pontikes, Y.; Santos, R.M.; Snellings, R.; Vandewalle, L.; Blanpain, B.; Van Balen, K. Effect of accelerated carbonation on AOD stainless steel slag for its valorisation as a CO2-sequestering construction material. Chem. Eng. J. 2014, 246, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Baciocchi, R.; Costa, G.; Bartolomeo, E.D.; Polettini, A.; Pomi, R. Carbonation of Stainless Steel Slag as a Process for CO2 Storage and Slag Valorization. Waste Biomass Valorization 2010, 1, 467–477. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Di Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.J.J.; Comans, R.N.J. Carbonation of steel slag for CO2 sequestration: Leaching of products and reaction mechanisms. Environ. Sci. Technol. 2006, 40, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.J.J.; Witkamp, G.J.; Comans, R. Mineral CO2 Sequestration by Steel Slag Carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zeng, Y.N.; Li, J.G.; Zhang, Y.Z.; Zhao, Q.Z. Carbonation of argon oxygen decarburization stainless steel slag and its effect on chromium leachability. J. Clean. Prod. 2020, 256, 120377. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zeng, Y.N.; Li, J.G.; Zhang, Y.Z. Cementitious behavior of argon oxygen decarburization stainless steel slag and its stabilization on chromium. Crystals 2020, 10, 876. [Google Scholar]
- Homma, S.; Ogata, S.; Koga, J.; Matsumoto, S. Gas–solid reaction model for a shrinking spherical particle with unreacted shrinking core. Chem. Eng. Sci. 2005, 60, 4971–4980. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering. Ind. Eng. Chem. Res. 1999, 38, 4140–4143. [Google Scholar] [CrossRef]
- Takasu, H.; Funayama, S.; Uchiyama, N.; Hoshino, H.; Tamura, Y.; Kato, Y. Kinetic analysis of the carbonation of lithium orthosilicate using the shrinking core model. Ceram. Int. 2018, 44, 11835–11839. [Google Scholar] [CrossRef]
- Cao, T.Y. Dynamic study on lime carbonation. Wuhan Iron Steel Corp. Technol. 2010, 48, 21–23. [Google Scholar] [CrossRef]
- Zeng, Y.N.; Ren, Q.Q.; Liu, B.; Li, J.G. Kinetic model of chromium release from argon oxygen decarburisation (AOD) slag in a neutral leachate. J. Iron Steel Res. Int. 2020, 27, 1303–1310. [Google Scholar] [CrossRef]











| Composition | CaO | SiO2 | MgO | Al2O3 | TiO2 | Cr2O3 | FeO | MnO | P2O5 | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Content | 65 | 23.93 | 6.36 | 1.94 | 1.20 | 0.31 | 1.15 | 0.09 | 0.002 | 0.06 |
| Composition | Ca2SiO4 | CaF2 | MgO | MgCr2O4 | Ca3Mg (SiO4)2 |
|---|---|---|---|---|---|
| Content | 77.6 | 12.8 | 9.8 | 0.2 | Microphase |
| Mineral Phase | Carbonation Reaction Equation | Log (K) | ΔrGθ (kJ/mol) | ΔrGθ = A + BT (kJ/mol) |
|---|---|---|---|---|
| Ca2SiO4 | Ca2SiO4(A) + 2CO2 = 2CaCO3 + SiO2 | 26.637 | −152.029 | 0.335T-160.538 |
| Ca2SiO4(AA) + 2CO2 = 2CaCO3 + SiO2 | 23.260 | −132.755 | 0.315T-140.773 | |
| Ca2SiO4(B) + 2CO2 = 2CaCO3 + SiO2 | 23.221 | −132.532 | 0.319T-140.650 | |
| Ca2SiO4(L) + 2CO2 = 2CaCO3 + SiO2 | 23.439 | −133.777 | 0.329T-142.019 | |
| Ca2SiO4(O) + 2CO2 = 2CaCO3 + SiO2 | 21.989 | −125.498 | 0.322T-133.559 | |
| MgO | MgO + CO2 = MgCO3 | 8.503 | −48.531 | 0.175T-52.918 |
| f-CaO | CaO + CO2 = CaCO3 | 22.852 | −130.423 | 0.160T-134.421 |
| Slag Sample | CaCO3 | Ca2SiO4 | CaF2 | CaCO3(A) |
|---|---|---|---|---|
| 60 °C | 50.5 | 38.1 | 11.4 | Microphase |
| 80 °C | 61.9 | 22.6 | 9.4 | 4.5 |
| 100 °C | 65.5 | 21.3 | 10.0 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, M.-J.; Wang, Y.-J.; Li, J.-G.; Zeng, Y.-N.; Liu, S.-H.; Qin, S. Slurry-Phase Carbonation Reaction Characteristics of AOD Stainless Steel Slag. Processes 2021, 9, 2266. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122266
Tao M-J, Wang Y-J, Li J-G, Zeng Y-N, Liu S-H, Qin S. Slurry-Phase Carbonation Reaction Characteristics of AOD Stainless Steel Slag. Processes. 2021; 9(12):2266. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122266
Chicago/Turabian StyleTao, Meng-Jie, Ya-Jun Wang, Jun-Guo Li, Ya-Nan Zeng, Shao-Hua Liu, and Song Qin. 2021. "Slurry-Phase Carbonation Reaction Characteristics of AOD Stainless Steel Slag" Processes 9, no. 12: 2266. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122266