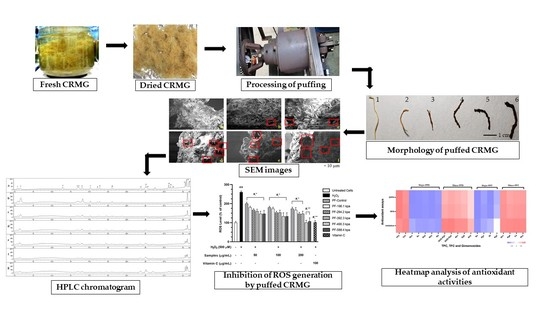
Ginsenosides Conversion and Anti-Oxidant Activities in Puffed Cultured Roots of Mountain Ginseng
Abstract
:1. Introduction
2. Material and Method
2.1. Material
2.2. Chemicals
2.3. Puffing of CRMG
2.4. Extraction Process
2.5. Crude Ginsenosides Content
2.6. Ginsenoside Analysis
2.7. Scanning Electron Microscope
2.8. Analysis of Total Phenolics
2.9. Analysis of Total Flavonoids
2.10. DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Radical Scavenging Activity
2.11. Reducing Power Test
2.12. Cytotoxic Effect of Puffed CRMG Extracts on HaCaT Cells
2.13. Effect of H2O2 on Cell Viability of HaCaT Cells
2.14. Effect of Puffed CRMG Extracts on ROS Production in HaCaT Cells under Oxidative Stress
2.15. Statistical Analysis
3. Results and Discussion
3.1. Morphological Characteristics of Puffed CRMG
3.2. Extraction Yield and Crude Ginsenoside Contents
3.3. Ginsenosides Analysis
3.4. TPC, TFC and Antioxidant Activities
3.5. Effect of Puffed CRMG Extracts on Cell Viability of HaCaT Cells
3.6. Effect of H2O2-Induced Oxidative Stress on Cell Viability of HaCaT Cells
3.7. Effect of Puffed CRMG Samples on ROS Production in H2O2-Induced Oxidative Stress-Treated HaCaT Cells
3.8. Correlation Map among Contents of Ginsenosides, TPC, TFC and Antioxidant Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.H. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012, 36, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.-D.; Chiu, C.-H.; Hsu, Y.-J.; Hou, C.-W.; Chen, Y.-M.; Huang, C.-C. Changbai Mountain ginseng (Panax ginseng CA Mey) extract supplementation improves exercise performance and energy utilization and decreases fatigue-associated parameters in mice. Molecules 2017, 22, 237. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Yang, Y.; Yang, X.; Guan, Y.; Yang, J.; Quan, J.; Yan, H.; Hou, W.; Zhang, G. Growth age of mountain cultivated ginseng affects its chemical composition. Ind. Crops Prod. 2021, 167, 113531. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Park, S.-Y.; Paek, K.-Y. Quality, safety and efficacy profiling of ginseng adventitious roots produced in vitro. Appl. Microbiol. Biotechnol. 2018, 102, 7309–7317. [Google Scholar] [CrossRef] [PubMed]
- Paek, K.-Y.; Murthy, H.N.; Hahn, E.-J.; Zhong, J.-J. Large scale culture of ginseng adventitious roots for production of ginsenosides. Biotechnol. China I 2009, 113, 151–176. [Google Scholar]
- Jiménez-Pérez, Z.E.; Kim, Y.-J.; Castro-Aceituno, V.; Mathiyalagan, R.; Markus, J.; Ahn, S.; Simu, S.Y.; Yang, D.-C. Novel application of cultured roots of mountain ginseng (Panax ginseng meyer) and ginsenoside re as safe antimelanogenic cosmeceutical components. Afr. J. Tradit. Complementary Altern. Med. 2017, 14, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Piao, X.M.; Huo, Y.; Kang, J.P.; Mathiyalagan, R.; Zhang, H.; Yang, D.U.; Kim, M.; Yang, D.C.; Kang, S.C.; Wang, Y.P. Diversity of ginsenoside profiles produced by various processing technologies. Molecules 2020, 25, 4390. [Google Scholar] [CrossRef]
- Yang, W.-Z.; Hu, Y.; Wu, W.-Y.; Ye, M.; Guo, D.-A. Saponins in the genus Panax L. (Araliaceae): A systematic review of their chemical diversity. Phytochemistry 2014, 106, 7–24. [Google Scholar] [CrossRef]
- Yang, W.; Qiao, X.; Li, K.; Fan, J.; Bo, T.; Guo, D.-a.; Ye, M. Identification and differentiation of Panax ginseng, Panax quinquefolium, and Panax notoginseng by monitoring multiple diagnostic chemical markers. Acta Pharm. Sin. B 2016, 6, 568–575. [Google Scholar] [CrossRef] [Green Version]
- Du, G.-J.; Dai, Q.; Williams, S.; Wang, C.-Z.; Yuan, C.-S. Synthesis of protopanaxadiol derivatives and evaluation of their anticancer activities. Anti-Cancer Drugs 2011, 22, 35. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-O.; Hwang, S.-H.; Shen, T.; Kim, J.H.; You, L.; Hu, W.; Cho, J.Y. Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J. Ginseng Res. 2021, 45, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Wang, H.; Qu, C.; Xie, L.; Wicks, S.M.; Xie, J. Ginsenoside Re: Its chemistry, metabolism and pharmacokinetics. Chin. Med. 2012, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nakhjavani, M.; Palethorpe, H.M.; Tomita, J.; Smith, E.; Price, T.J.; Yool, A.J.; Pei, J.V.; Townsend, A.R.; Hardingham, J.E. Stereoselective Anti-Cancer Activities of Ginsenoside Rg3 on Triple Negative Breast Cancer Cell Models. Pharmaceuticals 2019, 12, 117. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Liu, T.; Li, W.; Li, J.; Wang, C.; Zhang, K. Insights into the antitumor mechanism of ginsenosides Rg3. Mol. Biol. Rep. 2021, 48, 2639–2652. [Google Scholar] [CrossRef]
- Jin, J.P.; An, J.; Lee, J.D.; Kim, H.Y.; Kim, K.B. Effects of anti-wrinkle and skin-whitening fermented black ginseng on human subjects and underlying mechanism of action. J. Toxicol. Environ. Health Part A 2020, 83, 1–15. [Google Scholar]
- Song, Y.N.; Hong, H.G.; Son, J.S.; Kwon, Y.O.; Yoon, M.H. Investigation of Ginsenosides and Antioxidant Activities in the Roots, Leaves, and Stems of Hydroponic-Cultured Ginseng (Panax ginseng Meyer). Prev. Nutr. Food Sci. 2019, 24, 283–292. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.-W.; Li, W.; Ma, H.; Sun, J.; Deng, M.-C.; Yang, L. Ginsenoside metabolites, rather than naturally occurring ginsenosides, lead to inhibition of human cytochrome P450 enzymes. Toxicol. Sci. 2006, 91, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.S.; Gu, L.J.; Fang, Z.M.; Wang, C.Y.; Wang, Z.; Lee, M.R.; Zheng, L.; Li, J.J.; Sung, C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J. Pharm. Biomed. Anal. 2009, 50, 15–22. [Google Scholar] [CrossRef]
- Jin, Y.; Kim, Y.-J.; Jeon, J.-N.; Wang, C.; Min, J.-W.; Noh, H.-Y.; Yang, D.-C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods Hum. Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Subramani, D.; Tamilselvan, S.; Murugesan, M.; Shivaswamy, M. Optimization of Sand Puffing Characteristics of Quinoa using Response Surface Methodology. Curr. Res. Nutr. Food Sci. J. 2020, 8, 496–503. [Google Scholar] [CrossRef]
- Farh, M.E.-A.; Kim, Y.-J.; Abbai, R.; Singh, P.; Jung, K.-H.; Kim, Y.-J.; Yang, D.-C. Pathogenesis strategies and regulation of ginsenosides by two species of Ilyonectria in Panax ginseng: Power of speciation. J. Ginseng Res. 2020, 44, 332–340. [Google Scholar] [CrossRef]
- An, Y.E.; Ahn, S.C.; Yang, D.C.; Park, S.J.; Baik, M.Y. Chemical conversion of ginsenosides in puffed red ginseng. LWT—Food Sci. Technol. 2011, 44, 370–374. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Joung, E.M.; Woo, K.S.; Jeong, J.H.; Yu, K.W.; Lee, J.; Jeong, H.S. Changes in ginsenosides and antioxidant activity of Korean ginseng (Panax ginseng C.A. Meyer) with Heating Temperature and Pressure. Food Sci. Biotechnol. 2010, 19, 941–949. [Google Scholar] [CrossRef]
- Guo, H.; Saravanakumar, K.; Wang, M.-H. Total phenolic, flavonoid contents and free radical scavenging capacity of extracts from tubers of Stachys affinis. Biocatal. Agric. Biotechnol. 2018, 15, 235–239. [Google Scholar] [CrossRef]
- Shin, J.-H.; Park, Y.J.; Kim, W.; Kim, D.-O.; Kim, Y.; Hyungjae, L.; Baik, M.-Y. Change of Ginsenoside Profiles in Processed Ginseng by Drying, Steaming, and Puffing. J. Microbiol. Biotechnol. 2019, 29, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-S.; Jeon, S.-J.; Youn, S.J.; Lee, H.; Park, Y.-J.; Kim, D.-O.; Kim, B.-Y.; Kim, W.; Baik, M.-Y. Enhancement of minor ginsenosides contents and antioxidant capacity of american and canadian ginsengs (Panax quinquefolius) by puffing. Antioxidants 2019, 8, 527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, E.-M.; Park, J.; Song, H.-R.; Kim, J.-M.; Lee, M.; Song, H.-K.; Hong, O.-Y.; Whang, P.H.; Han, M.-K.; Kwon, K.-B. Skin aging-dependent activation of the PI3K signaling pathway via downregulation of PTEN increases intracellular ROS in human dermal fibroblasts. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Abid, S.; Kaliraj, L.; Rahimi, S.; Kim, Y.J.; Yang, D.C.; Kang, S.C.; Balusamy, S.R. Synthesis and characterization of glycol chitosan coated selenium nanoparticles acts synergistically to alleviate oxidative stress and increase ginsenoside content in Panax ginseng. Carbohydr. Polym. 2021, 267, 118195. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Yang, D.U.; Arunkumar, L.; Han, Y.; Lee, S.J.; Arif, M.H.; Li, J.F.; Huo, Y.; Kang, J.P.; Hoang, V.A. Cumulative Production of Bioactive Rg3, Rg5, Rk1, and CK from Fermented Black Ginseng Using Novel Aspergillus niger KHNT-1 Strain Isolated from Korean Traditional Food. Processes 2021, 9, 227. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.J.; Paek, K.Y. CO2-induced total phenolics in suspension cultures of Panax ginseng C. A. Mayer roots: Role of antioxidants and enzymes. Plant Physiol. Biochem. 2005, 43, 449–457. [Google Scholar] [CrossRef]
- Ffda, A.; Ddpf, A.; Nn, A.; Da, B.; Jd, B.; Fgds, A.; Rrc, B.; Ckds, C.; Gmp, A. Influence of high-intensity ultrasound on color, chemical composition and antioxidant properties of araá-boi pulp. Food Chem. 2021, 338, 127747. [Google Scholar]
- Su, Y.; Li, L. Structural Characterization and Antioxidant Activity of polysaccharide from four Auriculariales. Carbohydr. Polym. 2019, 229, 115407. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Nariya, P.B.; Acharya, R.N.; Shukla, V.J. In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula Linn. AYU 2013, 34, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warinhomhoun, S.; Muangnoi, C.; Buranasudja, V.; Mekboonsonglarp, W.; Rojsitthisak, P.; Likhitwitayawuid, K.; Sritularak, B. Antioxidant Activities and Protective Effects of Dendropachol, a New Bisbibenzyl Compound from Dendrobium pachyglossum, on Hydrogen Peroxide-Induced Oxidative Stress in HaCaT Keratinocytes. Antioxidants 2021, 10, 252. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.R.; Lee, G.D.; Park, J.H.; Lee, I.S.; Kwon, J.H. Ginsenoside composition and antiproliferative activities of explosively puffed ginseng (Panax ginseng CA Meyer). J. Food Sci. 2010, 75, C378–C382. [Google Scholar] [CrossRef]
- Choi, Y.; Ban, I.; Lee, H.; Baik, M.-Y.; Kim, W. Puffing as a novel process to enhance the antioxidant and anti-inflammatory properties of Curcuma longa L. (turmeric). Antioxidants 2019, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-W.; Noh, Y.-C.; Park, S.-Y.; Lee, M.; Choi, H.-J.; Song, G.-Y.; Kim, H. Effects of 20 (R)-Ginsenoside Rg3 and 20 (S)-Ginsenoside Rg3 on the migration and invasion of HaCaT cells. Yakhak Hoeji 2021, 65, 65–71. [Google Scholar] [CrossRef]
- Ahn, S.; Siddiqi, M.H.; Aceituno, V.C.; Simu, S.Y.; Zhang, J.; Perez, Z.E.J.; Kim, Y.-J.; Yang, D.-C. Ginsenoside Rg5: Rk1 attenuates TNF-α/IFN-γ-induced production of thymus-and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. Vitr. Cell. Dev. Biol.—Anim. 2016, 52, 287–295. [Google Scholar] [CrossRef]
- Lee, S.Y. Anti-metastatic and anti-inflammatory effects of matrix metalloproteinase inhibition by ginsenosides. Biomedicines 2021, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-R.; Kim, J.-H.; Kang, Y.-J.; Cho, S.-G. The anti-apoptotic and anti-oxidant effect of eriodictyol on UV-induced apoptosis in keratinocytes. Biol. Pharm. Bull. 2007, 30, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; You, D.H.; Han, T.; Choi, E.-M. Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). J. Photochem. Photobiol. B Biol. 2014, 141, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Lin, Y.-S.; Huang, Y.-W.; Fang, S.-U.; Lin, S.-Y.; Hou, W.-C. Protective effects of minor components of curcuminoids on hydrogen peroxide-treated human HaCaT keratinocytes. J. Agric. Food Chem. 2016, 64, 3598–3608. [Google Scholar] [CrossRef]
- Sritularak, B.; Likhitwitayawuid, K. New bisbibenzyls from Dendrobium falconeri. Helv. Chim. Acta 2009, 92, 740–744. [Google Scholar] [CrossRef]
- Ransy, C.; Vaz, C.; Lombès, A.; Bouillaud, F. Use of H2O2 to cause oxidative stress, the catalase issue. Int. J. Mol. Sci. 2020, 21, 9149. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, Y.M.; Song, S.; Lee, Y.Y.; Yeum, K.J. Black soybeans protect human keratinocytes from oxidative stress-induced cell death. Food Sci. Nutr. 2018, 6, 2423–2430. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Mao, X. Chitopentaose protects HaCaT cells against H2O2-induced oxidative damage through modulating MAPKs and Nrf2/ARE signaling pathways. J. Funct. Foods 2020, 72, 104086. [Google Scholar] [CrossRef]
- Choudhry, Q.N.; Kim, J.H.; Cho, H.T.; Heo, W.; Lee, J.-J.; Lee, J.H.; Kim, Y.J. Ameliorative effect of black ginseng extract against oxidative stress-induced cellular damages in mouse hepatocytes. J. Ginseng Res. 2019, 43, 179–185. [Google Scholar] [CrossRef]
- Laguerre, M.; Wrutniak-Cabello, C.; Chabi, B.; López Giraldo, L.J.; Lecomte, J.; Villeneuve, P.; Cabello, G. Does hydrophobicity always enhance antioxidant drugs? A cut-off effect of the chain length of functionalized chlorogenate esters on ROS-overexpressing fibroblasts. J. Pharm. Pharmacol. 2011, 63, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Pan, X.; Lv, J.; Zhong, W.; Yan, F.; Duan, F.; Jia, L. Effects of explosion puffing on the nutritional composition and digestibility of grains. Int. J. Food Prop. 2018, 21, 2193–2204. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Benabderrahim, M.A.; Yahia, Y.; Bettaieb, I.; Elfalleh, W.; Nagaz, K. Antioxidant activity and phenolic profile of a collection of medicinal plants from Tunisian arid and Saharan regions. Ind. Crops Prod. 2019, 138, 111427. [Google Scholar] [CrossRef]
- Park, S.K.; Hyun, S.H.; In, G.; Park, C.-K.; Kwak, Y.-S.; Jang, Y.-J.; Kim, B.; Kim, J.-H.; Han, C.-K. The antioxidant activities of Korean Red Ginseng (Panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. J. Ginseng Res. 2021, 45, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Kang, K.A.; Youn, U.; Park, J.S.; Hyun, J.W. A comparative study of the potential antioxidant activities of ginsenosides. J. Food Biochem. 2010, 34, 31–43. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, M.Y.; Reddy, C.K.; Lee, S.J.; Lee, C.H. Evaluation of Metabolite Profiles of Ginseng Berry Pomace Obtained after Different Pressure Treatments and Their Correlation with the Antioxidant Activity. Molecules 2021, 26, 284. [Google Scholar] [CrossRef] [PubMed]









| Puffed CRMG | Minor Ginsenosides | Total Major Ginsenosides | Total Ginsenosides | Extraction Yield | Crude Ginsenosides | ||
|---|---|---|---|---|---|---|---|
| Minor-PPD | Minor-PPT | Total Minor | |||||
| mg/g DW | mg/g DW | mg/g DW | mg/g DW | mg/g DW | mg/g DW | mg/g DW | |
| Control | ND | ND | ND | 10.36 ± 0.42 a | 10.36 ± 0.42 a | 403.43 ± 26.61 a | 85.23 ± 5.25 a |
| 196.1 kpa | ND | 0.34 ± 0.02 d | 0.35 ± 0.02 e | 5.74 ± 0.13 b | 6.09 ± 0.14 bc | 385.07 ± 12.23 bc | 66.87 ± 4.07 d |
| 294.2 kpa | 0.06 ± 0 d | 0.38 ± 0.02 d | 0.45 ± 0.02 d | 5.02 ± 0.16 c | 5.46 ± 0.14 d | 372.63 ± 14.81 c | 63.33 ± 2.7 d |
| 392.3 kpa | 0.31 ± 0.02 c | 0.93 ± 0.05 c | 1.24 ± 0.04 c | 4.48 ± 0.1 d | 5.72 ± 0.13 cd | 360.77 ± 22.34 c | 71.17 ± 4.34 cd |
| 490.3 kpa | 0.66 ± 0.02 b | 1.33 ± 0.03 b | 2 ± 0.05 b | 3.74 ± 0.08 e | 5.74 ± 0.07 cd | 385.57 ± 15.6 bc | 78.67 ± 2.8 c |
| 588.4 kpa | 1.18 ± 0.05 a | 2.01 ± 0.06 a | 3.18 ± 0.06 a | 3.11 ± 0.05 f | 6.3 ± 0.05 b | 409.03 ± 15.72 b | 88.57 ± 5.72 b |
| Puffed CRMG | Contents of PPD-Type Ginsenosides (mg/g DW) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rb1 | Rc | Rb2 | Rb3 | Rd | 20(S)-Rg3 | 20(R)-Rg3 | Rk1 | Rg5 | Rh2 | |
| Control | 1.12 ± 0.07 a | 0.75 ± 0.03 a | 0.99 ± 0.06 a | 0.45 ± 0.04 a | 0.51 ± 0.03 a | ND | ND | ND | ND | ND |
| 196.1 kpa | 0.64 ± 0.03 b | 0.51 ± 0.04 b | 0.8 ± 0.03 b | 0.33 ± 0.01 b | 0.4 ± 0.01 b | ND | ND | ND | ND | ND |
| 294.2 kpa | 0.63 ± 0.03 bc | 0.41 ± 0.03 c | 0.72 ± 0.04 c | 0.3 ± 0 bc | 0.38 ± 0.02 b | 0.01 ± 0 d | 0.01 ± 0 d | 0.05 ± 0 d | ND | ND |
| 392.3 kpa | 0.61 ± 0.04 bc | 0.36 ± 0.02 d | 0.69 ± 0.02 cd | 0.28 ± 0.01 c | 0.29 ± 0.01 c | 0.04 ± 0 c | 0.02 ± 0 c | 0.22 ± 0.02 c | 0.04 ± 0 c | ND |
| 490.3 kpa | 0.56 ± 0.03 c | 0.31 ± 0.01 e | 0.64 ± 0.03 d | 0.26 ± 0.02 c | 0.28 ± 0.03 c | 0.13 ± 0.01 b | 0.04 ± 0 b | 0.35 ± 0.02 b | 0.14 ± 0.01 b | ND |
| 588.4 kpa | 0.47 ± 0.02 d | 0.22 ± 0.01 f | 0.52 ± 0.02 e | 0.21 ± 0.01 d | 0.22 ± 0.01 d | 0.18 ± 0.02 a | 0.05 ± 0 a | 0.64 ± 0.04 a | 0.28 ± 0.02 a | 0.02 ± 0 a |
| Puffed CRMG | Contents of PPT-Type Ginsenosides (mg/g DW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rg1 | Re | Rf | Rg2 | Rh1 | Rg6 | F4 | Rk3 | PPT | |
| Control | 0.63 ± 0.07 a | 5.31 ± 0.35 a | 0.16 ± 0.02 a | 0.33 ± 0.01 a | 0.09 ± 0 c | ND | ND | ND | ND |
| 196.1 kpa | 0.56 ± 0.01 b | 2.08 ± 0.11 b | 0.11 ± 0 b | 0.21 ± 0.01 e | 0.1 ± 0 bc | 0.13 ± 0.01 d | 0.13 ± 0.01 d | 0.09 ± 0.01 d | ND |
| 294.2 kpa | 0.47 ± 0.05 c | 1.67 ± 0.04 c | 0.11 ± 0 b | 0.22 ± 0.01 de | 0.1 ± 0 bc | 0.14 ± 0.02 d | 0.15 ± 0.01 d | 0.09 ± 0.01 d | ND |
| 392.3 kpa | 0.42 ± 0.01 c | 1.39 ± 0.07 c | 0.11 ± 0 b | 0.23 ± 0.01 d | 0.1 ± 0 b | 0.38 ± 0.01 c | 0.34 ± 0.02 c | 0.21 ± 0.01 c | ND |
| 490.3 kpa | 0.32 ± 0.01 d | 0.9 ± 0.08 d | 0.1 ± 0 b | 0.26 ± 0.01 c | 0.11 ± 0 ab | 0.55 ± 0.03 b | 0.49 ± 0.01 b | 0.29 ± 0.01 b | ND |
| 588.4 kpa | 0.26 ± 0.01 d | 0.71 ± 0.03 d | 0.1 ± 0 b | 0.29 ± 0.01 b | 0.11 ± 0 a | 0.87 ± 0.03 a | 0.71 ± 0.03 a | 0.43 ± 0.02 a | ND |
| Puffed CRMG | TPC | TFC | In Vitro Antioxidant | |
|---|---|---|---|---|
| DPPH | RPA | |||
| mg GAE/g DW | mg RE/g DW | mg GAE/g DW | mg GAE/g DW | |
| Control | 3.85 ± 0.12 f | 3.4 ± 0.03 e | 0.18 ± 0.05 d | 1.37 ± 0.01 h |
| 196.1 kpa | 8.41 ± 0.21 e | 4.3 ± 0.15 d | 1.31 ± 0.04 c | 3.82 ± 0.09 e |
| 294.2 kpa | 8.99 ± 0.27 d | 4.44 ± 0.14 d | 1.45 ± 0.03 b | 4.23 ± 0.13 c |
| 392.3 kpa | 9.94 ± 0.38 c | 5.07 ± 0.03 c | 1.51 ± 0.02 b | 4.56 ± 0.19 b |
| 490.3 kpa | 11.14 ± 0.33 b | 5.76 ± 0.05 b | 1.69 ± 0.02 a | 5.22 ± 0.19 a |
| 588.4 kpa | 11.81 ± 0.26 a | 6.43 ± 0.11 a | 1.66 ± 0.01 a | 4.95 ± 0.31 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, J.Y.; Ramadhania, Z.M.; Mathiyalagan, R.; Huo, Y.; Han, Y.; Li, J.F.; Ahn, J.C.; Xu, F.J.; Lee, D.W.; Zeng, X.H.; et al. Ginsenosides Conversion and Anti-Oxidant Activities in Puffed Cultured Roots of Mountain Ginseng. Processes 2021, 9, 2271. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122271
Pu JY, Ramadhania ZM, Mathiyalagan R, Huo Y, Han Y, Li JF, Ahn JC, Xu FJ, Lee DW, Zeng XH, et al. Ginsenosides Conversion and Anti-Oxidant Activities in Puffed Cultured Roots of Mountain Ginseng. Processes. 2021; 9(12):2271. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122271
Chicago/Turabian StylePu, Jian Yu, Zelika Mega Ramadhania, Ramya Mathiyalagan, Yue Huo, Yaxi Han, Jin Feng Li, Jong Chan Ahn, Feng Jiao Xu, Dong Wook Lee, Xu Hui Zeng, and et al. 2021. "Ginsenosides Conversion and Anti-Oxidant Activities in Puffed Cultured Roots of Mountain Ginseng" Processes 9, no. 12: 2271. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122271