Catalytic Activity of Ni Nanotubes Covered with Nanostructured Gold
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
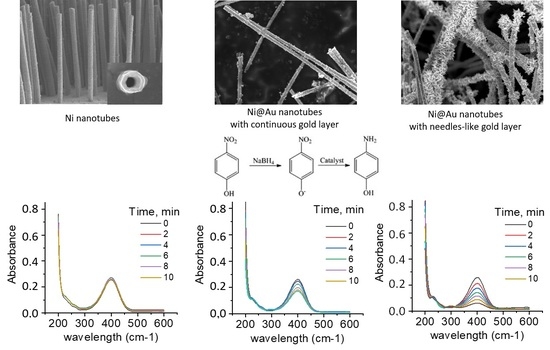
- A simple and well-controlled chemical reaction of the transformation of substance A into B should be observed in the presence of nanostructures, and the termination of the reaction without them;
- Analysis of reaction kinetics must be performed by simple and accurate methods (e.g., UV–Vis spectroscopy), and the reaction mechanism, including a possible intermediate state, must be precisely known;
- The reaction must proceed under rather mild conditions in order to avoid any side reactions or possible dissolution of the nanoparticle.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter. 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Campbell, C.T. The active site in nanoparticle gold catalysis. Science 2004, 306, 234–235. [Google Scholar] [CrossRef]
- Son, H.Y.; Kim, K.R.; Hong, C.A.; Nam, Y.S. Morphological evolution of gold nanoparticles into nanodendrites using catechol-grafted polymer templates. ACS Omega 2018, 3, 6683–6691. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Lin, C.; Tao, K.; Hua, D.; Ma, Z.; Zhou, S. Size effect of gold nanoparticles in catalytic reduction of p-nitrophenol with NaBH4. Molecules 2013, 18, 12609–12620. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Li, Y. Introduction to Surface Chemistry and Catalysis; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Alam, M.N.; Batuta, S.; Ahamed, G.; Das, S.; Mandal, D.; Begum, N.A. Tailoring the catalytic activity of Au nanoparticles synthesized by a naturally occurring green multifunctional agent. Arab. J. Chem. 2019, 12, 3825–3835. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Sun, Y.; Huang, J.; Liang, Z.; Li, S.; Jiang, L. Morphological effects on the selectivity of intramolecular: Versus intermolecular catalytic reaction on Au nanoparticles. Nanoscale 2017, 9, 7727–7733. [Google Scholar] [CrossRef]
- Wu, B.; Kuang, Y.; Zhang, X.; Chen, J. Noble metal nanoparticles/carbon nanotubes nanohybrids: Synthesis and applications. Nano Today 2011, 6, 75–90. [Google Scholar] [CrossRef]
- Alshammari, H.M.; Alshammari, A.S.; Humaidi, J.R.; Alzahrani, S.A.; Alhumaimess, M.S.; Aldosari, O.F.; Hassan, H.M.A. Au-Pd bimetallic nanocatalysts incorporated into carbon nanotubes (CNTs) for selective oxidation of alkenes and alcohol. Processes 2020, 8, 1380. [Google Scholar] [CrossRef]
- Kazemi, M. Based on magnetic nanoparticles: Gold reusable nanomagnetic catalysts in organic synthesis. Synth. Commun. 2020, 50, 2079–2094. [Google Scholar] [CrossRef]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Mitchell, D.T.; Lee, S.B.; Trofin, L.; Li, N.; Nevanen, T.K.; Soderland, H.; Martin, C.R. Smart Nanotubes for Bioseparations and Biocatalysis. J. Am. Chem. Soc. 2002, 124, 11864–11865. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Shlimas, D.I.; Kozlovskiy, A.L.; Zdorovets, M.V.; Russakova, A.V.; Kassymzhanov, M.; Borisenko, A.N. Electron Beam Induced Enhancement of the Catalytic Properties of Ion-Track Membranes Supported Copper Nanotubes in the Reaction of the P-Nitrophenol Reduction. Catalysts 2019, 9, 737. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Tan, F.; Wang, W.; Qiu, X.; Qiao, X.; Chen, J. Facile, template-free synthesis of silver nanodendrites with high catalytic activity for the reduction of p-nitrophenol. J. Hazard. Mater. 2012, 217–218, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, H.M.; Humaidi, J.R.; Alhumaimess, M.S.; Aldosari, O.F.; Alotaibi, M.H.; Hassan, H.M.A.; Wawata, I. Bimetallic Au:Pd nanoparticle supported on MgO for the oxidation of benzyl alcohol. Reac. Kinet. Mech. Cat. 2019, 128, 97–108. [Google Scholar] [CrossRef]
- Thongthai, K.; Srisombat, L.; Saipanya, S.; Ananta, S. Morphology and atalytic activity of gold core-platinum shell nanoparticles. Chiang Mai J. Sci. 2015, 42, 481–489. [Google Scholar]
- Roselina, N.R.; Azizan, A.; Hyie, K.M.; Murad, M.C.; Abdullah, A.H. Synthesis and characterization of Ni-Au bimetallic nanoparticles. Int. J. Mod. Phys. B 2015, 29, 1540006. [Google Scholar] [CrossRef]
- Ahmad, T.; Bae, H.; Rhee, I.; Chang, Y.; Jin, S.-U.; Hong, S. Gold-coated iron oxide nanoparticles as a contrast agent in magnetic resonance imaging. J. Nanosci. Nanotechnol. 2012, 12, 5132–5137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, S.M.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef] [Green Version]
- Kozlovskiy, A.; Zdorovets, M.; Shumskaya, A.; Kanyukov, E.; Kutuzau, M. Synthesis and Properties of Ni x/Au 1-x Nanotubes. In Proceedings of the 2017 IEEE 7th International Conference Nanomaterials: Application & Properties (NAP), Odessa, Ukraine, 10–15 September 2017; pp. 04NB21-1–04NB21-3. [Google Scholar]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Gorin, Y.G.; Kozlovskiy, A.L.; Zdorovets, M.V.; Zhidkov, I.S.; Cholach, S.O. Electron/gamma radiation-induced synthesis and catalytic activity of gold nanoparticles supported on track-etched poly(ethylene terephthalate) membranes. Mater. Chem. Phys. 2018, 217, 31–39. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Ibragimova, M.A.; Akhmetova, S.B.; Kozlovskiy, A.L. Synthesis, radical scavenging, and antimicrobial activities of core–shell Au / Ni microtubes. Chem. Pap. 2020, 74, 2189–2199. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts. 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Pondman, K.M.; Maijenburg, A.W.; Celikkol, F.B.; Pathan, A.A.; Kishore, U.; ten Haken, B.; ten Elshof, J.E. Au coated Ni nanowires with tuneable dimensions for biomedical applications. J. Mater. Chem. B 2013, 1, 6129–6136. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhao, Z.; Zhang, M. Synthesis by Replacement Reaction and Application of TiO2-Supported Au-Ni Bimetallic Catalyst. ChemCatChem 2010, 2, 1606–1614. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Keane, M.A. Gas phase selective hydrogenation over oxide supported Ni-Au. Phys. Chem. Chem. Phys. 2015, 17, 28088–28095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruppert, A.M.; Jȩdrzejczyk, M.; Potrzebowska, N.; Kaźmierczak, K.; Brzezińska, M.; Sneka-Płatek, O.; Sautet, P.; Keller, N.; Michel, C.; Grams, J. Supported gold-nickel nano-alloy as a highly efficient catalyst in levulinic acid hydrogenation with formic acid as an internal hydrogen source. Catal. Sci. Technol. 2018, 8, 4318–4331. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Yang, X.; Zhang, J. Well-dispersed MgAl2O4 supported Ni catalyst with enhanced catalytic performance and the reason of its deactivation for long-term dry methanation reaction. Catalysts 2021, 11, 1117. [Google Scholar] [CrossRef]
- Chin, Y.H.; King, D.L.; Roh, H.S.; Wang, Y.; Heald, S.M. Structure and reactivity investigations on supported bimetallic Au{single bond}Ni catalysts used for hydrocarbon steam reforming. J. Catal. 2006, 244, 153–162. [Google Scholar] [CrossRef]
- Kaniukov, E.Y.; Shumskaya, E.E.; Yakimchuk, D.V.; Kozlovskiy, A.L.; Ibragimova, M.A.; Zdorovets, M.V. Evolution of the polyethylene terephthalate track membranes parameters at the etching process. J. Contem. Phys. 2017, 52, 155–160. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Borgekov, K.; Zdorovets, M.; Arkhangelsky, E.; Shumskaya, A.; Kanukov, E. Application of ion-track membranes in processes of direct and reverse osmosis. Proc. Natl. Acad. Sci. Belarus 2017, 1, 45–51. [Google Scholar]
- Korolkov, I.V.; Mashentseva, A.A.; Güven, O.; Zdorovets, M.V.; Taltenov, A.A. Enhancing hydrophilicity and water permeability of PET track-etched membranes by advanced oxidation process. Nucl. Instrum. Meth. Phys. Res. B 2015, 365, 651–655. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Güven, O.; Mashentseva, A.A.; Atıcı, A.B.; Gorin, Y.G.; Zdorovets, M.V.; Taltenov, A.A. Radiation induced deposition of copper nanoparticles inside the nanochannels of poly(acrylic acid)-grafted poly(ethylene terephthalate) track-etched membranes. Radiat. Phys. Chem. 2017, 130, 480–487. [Google Scholar] [CrossRef]
- Kaniukov, E.; Kozlovsky, A.; Shlimas, D.; Yakimchuk, D.; Zdorovets, M.; Kadyrzhanov, K. Tunable synthesis of copper nanotubes. IOP Conf. Ser. Mater. Sci. Eng. 2016, 110, 012013. [Google Scholar] [CrossRef] [Green Version]
- Kalkabay, G.; Kozlovskiy, A.; Zdorovets, M.; Borgekov, D.; Kaniukov, E.; Shumskaya, A. Influence of temperature and electrodeposition potential on structure and magnetic properties of nickel nanotubes. J. Magn. Magn. Mater. 2019, 489, 165436. [Google Scholar] [CrossRef]
- Shumskaya, A.; Bundyukova, V.; Kozlovskiy, A.; Zdorovets, M.; Kadyrzhanov, K.; Kalkabay, G.; Kaniukov, E. Evolution of morphology, structure, and magnetic parameters of Ni nanotubes with growth in pores of a PET template. J. Magn. Magn. Mater. 2020, 497, 165913. [Google Scholar] [CrossRef]
- Shumskaya, A.; Korolkov, I.; Rogachev, A.; Ignatovich, Zh.; Kozlovskiy, A.; Zdorovets, M.; Anisovich, M.; Bashouti, M.; Shalabny, A.; Busool, R.; et al. Synthesis of Ni@Au core-shell magnetic nanotubes for bioapplication and SERS detection. Colloids Surf. A Physicochem. Eng. 2021, 626, 127077. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Ludzik, K.; Kozlovskiy, A.L.; Fadeev, M.S.; Shumskaya, A.E.; Gorin, Y.G.; Marciniak, B.; Jazdzewska, M.; Chudoba, D.; Kontek, R.; et al. Carboranes immobilization on Fe3O4 nanocomposites for targeted delivery. Mater. Today Commun. 2020, 24, 101247. [Google Scholar] [CrossRef]
- Ciganda, R.; Li, N.; Deraedt, C.; Gatard, S.; Zhao, P. Gold nanoparticles as electron reservoir redox catalysts for 4-nitrophenol reduction: A strong stereoelectronic ligand influence. ChemComm. 2014, 50, 10126–10129. [Google Scholar] [CrossRef]
- Kuroda, K.; Ishida, T.; Haruta, M. Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J. Mol. Catal. A Chem. 2009, 298, 7–11. [Google Scholar] [CrossRef]







| Phase | (hkl) | 2θ° | d, Å | Crystallite Size, nm | Lattice Parameter, Å | FWHM |
|---|---|---|---|---|---|---|
| Ni-Cubic Fm-3m(225) | 111 | 44.586 | 2.03015 | 17.35 | 3.51760 | 0.550 |
| 200 | 51.835 | 1.76354 | 15.68 | 0.629 | ||
| 220 | 76.601 | 1.24424 | 17.67 | 0.636 |
| Ni@Au NTs, Concentration of Au in Solution | Phase | Crystallites Size, nm | Lattice Parameter, Å | Phase Concentration, % |
|---|---|---|---|---|
| 0.005 M | Ni-Cubic Fm-3m(225) | 19.87 ± 2.01 | 3.51991 | 79.5 |
| Au-cubic Fm-3m(225) | 12.29 ± 1.15 | 4.0618 | 20.5 | |
| 0.01 M | Ni-Cubic, Fm-3m(225) | 21.08 ± 1.61 | 3.53442 | 61.1 |
| Au-Cubic, Fm-3m(225) | 16.6 ± 1.35 | 4.08243 | 38.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumskaya, A.; Panina, L.; Rogachev, A.; Ihnatovich, Z.; Kozlovskiy, A.; Zdorovets, M.; Kaniukov, E.; Korolkov, I. Catalytic Activity of Ni Nanotubes Covered with Nanostructured Gold. Processes 2021, 9, 2279. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122279
Shumskaya A, Panina L, Rogachev A, Ihnatovich Z, Kozlovskiy A, Zdorovets M, Kaniukov E, Korolkov I. Catalytic Activity of Ni Nanotubes Covered with Nanostructured Gold. Processes. 2021; 9(12):2279. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122279
Chicago/Turabian StyleShumskaya, Alena, Larissa Panina, Alexander Rogachev, Zhanna Ihnatovich, Artem Kozlovskiy, Maxim Zdorovets, Egor Kaniukov, and Ilya Korolkov. 2021. "Catalytic Activity of Ni Nanotubes Covered with Nanostructured Gold" Processes 9, no. 12: 2279. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122279