Automatic Detection of Open and Vegetated Water Bodies Using Sentinel 1 to Map African Malaria Vector Mosquito Breeding Habitats
Abstract
:1. Introduction
2. Materials and Methods
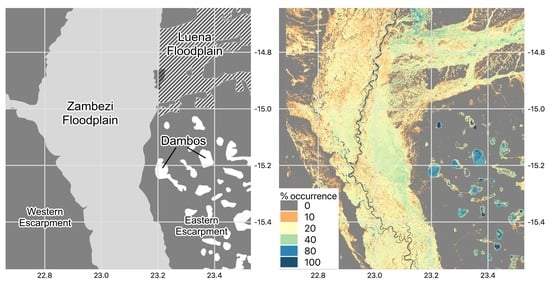
2.1. Study Site
2.2. Datasets and Classification Procedure
3. Results
4. Discussion
4.1. Water Body Detection
4.2. Hydrological Findings and Implications for Public Health
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bioresita, F.; Puissant, A.; Stumpf, A.; Malet, J.-P. A Method for Automatic and Rapid Mapping of Water Surfaces from Sentinel-1 Imagery. Remote Sens. 2018, 10, 217. [Google Scholar] [CrossRef]
- Westerhoff, R.S.; Kleuskens, M.P.H.; Winsemius, H.C.; Huizinga, H.J.; Brakenridge, G.R.; Bishop, C. Automated global water mapping based on wide-swath orbital synthetic-aperture radar. Hydrol. Earth Syst. Sci. 2013, 17, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Martinis, S.; Kersten, J.; Twele, A. A fully automated TerraSAR-X based flood service. ISPRS J. Photogramm. Remote Sens. 2015, 104, 203–212. [Google Scholar] [CrossRef]
- Pulvirenti, L.; Pierdicca, N.; Chini, M.; Guerriero, L. An algorithm for operational flood mapping from Synthetic Aperture Radar (SAR) data using fuzzy logic. Nat. Hazards Earth Syst. Sci. 2011, 11, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Giustarini, L.; Hostache, R.; Matgen, P.; Schumann, G.J.-P.; Bates, P.D.; Mason, D.C. A Change Detection Approach to Flood Mapping in Urban Areas Using TerraSAR-X. IEEE Trans. Geosci. Remote Sens. 2013, 51, 2417–2430. [Google Scholar] [CrossRef]
- Behnamian, A.; Banks, S.; White, L.; Brisco, B.; Millard, K.; Pasher, J.; Chen, Z.; Duffe, J.; Bourgeau-Chavez, L.; Battaglia, M. Semi-Automated Surface Water Detection with Synthetic Aperture Radar Data: A Wetland Case Study. Remote Sens. 2017, 9, 1209. [Google Scholar] [CrossRef]
- Schlaffer, S.; Chini, M.; Dettmering, D.; Wagner, W. Mapping Wetlands in Zambia Using Seasonal Backscatter Signatures Derived from ENVISAT ASAR Time Series. Remote Sens. 2016, 8, 402. [Google Scholar] [CrossRef]
- Clewley, D.; Whitcomb, J.; Moghaddam, M.; McDonald, K.; Chapman, B.; Bunting, P. Evaluation of ALOS PALSAR Data for High-Resolution Mapping of Vegetated Wetlands in Alaska. Remote Sens. 2015, 7, 7272–7297. [Google Scholar] [CrossRef] [Green Version]
- Brisco, B.; Li, K.; Tedford, B.; Charbonneau, F.; Yun, S.; Murnaghan, K. Compact polarimetry assessment for rice and wetland mapping. Int. J. Remote Sens. 2013, 34, 1949–1964. [Google Scholar] [CrossRef]
- Bwangoy, J.-R.B.; Hansen, M.C.; Roy, D.P.; De Grandi, G.; Justice, C.O. Wetland mapping in the Congo Basin using optical and radar remotely sensed data and derived topographical indices. Remote Sens. Environ. 2010, 114, 73–86. [Google Scholar] [CrossRef] [Green Version]
- WHO World Malaria Report 2018; WHO: Geneva, Switzerland, 2018.
- Bøgh, C.; Lindsay, S.W.; Clarke, S.E.; Dean, A.; Jawara, M.; Pinder, M.; Thomas, C.J. High spatial resolution mapping of malaria transmission risk in the Gambia, West Africa, using Landsat TM satellite imagery. Am. J. Trop. Med. Hyg. 2007, 76, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Mutuku, F.M.; Bayoh, M.N.; Hightower, A.W.; Vulule, J.M.; Gimnig, J.E.; Mueke, J.M.; Amimo, F.A.; Walker, E.D. A supervised land cover classification of a western Kenya lowland endemic for human malaria: Associations of land cover with larval Anopheles habitats. Int. J. Health Geogr. 2009, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Mushinzimana, E.; Munga, S.; Minakawa, N.; Li, L.; Feng, C.; Bian, L.; Kitron, U.; Schmidt, C.; Beck, L.; Zhou, G.; et al. Landscape determinants and remote sensing of anopheline mosquito larval habitats in the western Kenya highlands. Malar. J. 2006, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Pope, K.; Masuoka, P.; Rejmankova, E.; Grieco, J.; Johnson, S.; Roberts, D. Mosquito habitats, land use, and malaria risk in Belize from satellite imagery. Ecol. Appl. 2005, 15, 1223–1232. [Google Scholar] [CrossRef]
- Fillinger, U.; Lindsay, S.W. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop. Med. Int. Heal. 2006, 11, 1629–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killeen, G.F.; Fillinger, U.; Kiche, I.; Gouagna, L.C.; Knols, B.G.J. Eradication of Anopheles gambiae from Brazil: Lessons for malaria control in Africa? Lancet Infect. Dis. 2002, 2, 618–627. [Google Scholar] [CrossRef]
- Smith, M.W.; Macklin, M.G.; Thomas, C.J. Hydrological and geomorphological controls of malaria transmission. Earth Sci. Rev. 2013, 116, 109–127. [Google Scholar] [CrossRef]
- Smith, D.L.; Perkins, T.A.; Tusting, L.S.; Scott, T.W.; Lindsay, S.W. Mosquito Population Regulation and Larval Source Management in Heterogeneous Environments. PLoS ONE 2013, 8, e71247. [Google Scholar] [CrossRef] [PubMed]
- Catry, T.; Li, Z.; Roux, E.; Herbreteau, V.; Gurgel, H.; Mangeas, M.; Seyler, F.; Dessay, N. Wetlands and Malaria in the Amazon: Guidelines for the Use of Synthetic Aperture Radar Remote-Sensing. Int. J. Environ. Res. Public Health 2018, 15, 468. [Google Scholar] [CrossRef]
- Clement, M.A.; Kilsby, C.G.; Moore, P. Multi-temporal synthetic aperture radar flood mapping using change detection. J. Flood Risk Manag. 2018, 11, 152–168. [Google Scholar] [CrossRef]
- Santoro, M.; Wegmuller, U. Multi-temporal Synthetic Aperture Radar Metrics Applied to Map Open Water Bodies. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 3225–3238. [Google Scholar] [CrossRef]
- Charlwood, J.D.; Vij, R.; Billingsley, P.F. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of East Africa. Am. J. Trop. Med. Hyg. 2000, 62, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.C.; Davenport, I.J.; Neal, J.C.; Schumann, G.J.-P.; Bates, P.D. Near Real-Time Flood Detection in Urban and Rural Areas Using High-Resolution Synthetic Aperture Radar Images. IEEE Trans. Geosci. Remote Sens. 2012, 50, 3041–3052. [Google Scholar] [CrossRef]
- Chini, M.; Hostache, R.; Giustarini, L.; Matgen, P. A Hierarchical Split-Based Approach for Parametric Thresholding of SAR Images: Flood Inundation as a Test Case. IEEE Trans. Geosci. Remote Sens. 2017, 55, 6975–6988. [Google Scholar] [CrossRef]
- Matgen, P.; Hostache, R.; Schumann, G.; Pfister, L.; Hoffmann, L.; Savenije, H.H.G. Towards an automated SAR-based flood monitoring system: Lessons learned from two case studies. Phys. Chem. Earth Parts A/B/C 2011, 36, 241–252. [Google Scholar] [CrossRef]
- Martinis, S.; Plank, S.; Ćwik, K. The Use of Sentinel-1 Time-Series Data to Improve Flood Monitoring in Arid Areas. Remote Sens. 2018, 10, 583. [Google Scholar] [CrossRef]
- Martinis, S.; Twele, A.; Voigt, S. Towards operational near real-time flood detection using a split-based automatic thresholding procedure on high resolution TerraSAR-X data. Nat. Hazards Earth Syst. Sci. 2009, 9, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Bovolo, F.; Bruzzone, L. A Split-Based Approach to Unsupervised Change Detection in Large-Size Multitemporal Images: Application to Tsunami-Damage Assessment. IEEE Trans. Geosci. Remote Sens. 2007, 45, 1658–1670. [Google Scholar] [CrossRef]
- Huang, W.; DeVries, B.; Huang, C.; Lang, M.; Jones, J.; Creed, I.; Carroll, M. Automated Extraction of Surface Water Extent from Sentinel-1 Data. Remote Sens. 2018, 10, 797. [Google Scholar] [CrossRef]
- Hardy, A.; Makame, M.; Cross, D.; Majambere, S.; Msellem, M. Using low-cost drones to map malaria vector habitats. Parasites Vectors 2017, 10. [Google Scholar] [CrossRef]
- Hardy, A.J.; Mageni, Z.; Dongus, S.; Killeen, G.; Macklin, M.; Majambere, S.; Ali, A.S.; Smith, M.; Al-Mafazy, A.; Msellem, M.; et al. Mapping hotspots of malaria transmission from pre-existing hydrology, geology and geomorphology data in the pre-elimination context of Zanzibar, United Republic of Tanzania. Parasites Vectors 2015, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Dongus, S.; Nyika, D.; Kannady, K.; Mtasiwa, D.; Mshinda, H.; Gosoniu, L.; Drescher, A.W.; Fillinger, U.; Tanner, M.; Killeen, G.F. Urban agriculture and Anopheles habitats in Dar es Salaam, Tanzania. Geospat. Health 2009, 3, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Mwangangi, J.M.; Shililu, J.; Muturi, E.J.; Muriu, S.; Jacob, B.; Kabiru, E.W.; Mbogo, C.M.; Githure, J.; Novak, R.J. Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malar. J. 2010, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Hardy, A.J.; Gamarra, J.G.P.; Cross, D.E.; Macklin, M.G.; Smith, M.W.; Kihonda, J.; Killeen, G.F.; Ling’ala, G.N.; Thomas, C.J. Habitat Hydrology and Geomorphology Control the Distribution of Malaria Vector Larvae in Rural Africa. PLoS ONE 2013, 8, e81931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndenga, B.A.; Simbauni, J.A.; Mbugi, J.P.; Githeko, A.K.; Fillinger, U. Productivity of Malaria Vectors from Different Habitat Types in the Western Kenya Highlands. PLoS ONE 2011, 6, e19473. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, N.; Mutero, C.; Githure, J.; Beier, J.; Yan, G. Spatial distribution and habitat characterization of anopheline mosquito larvae in Western Kenya. Am. J. Trop. Med. Hyg. 1999, 61, 1010–1016. [Google Scholar] [CrossRef]
- Kwoun, O.; Lu, Z. Multi-temporal RADARSAT-1 and ERS Backscattering Signatures of Coastal Wetlands in Southeastern Louisiana. Photogramm. Eng. Remote Sens. 2009, 75, 607–617. [Google Scholar] [CrossRef]
- Moser, L.; Schmitt, A.; Wendleder, A.; Roth, A.; Moser, L.; Schmitt, A.; Wendleder, A.; Roth, A. Monitoring of the Lac Bam Wetland Extent Using Dual-Polarized X-Band SAR Data. Remote Sens. 2016, 8, 302. [Google Scholar] [CrossRef]
- Dobson, M.C.; Ulaby, F.T.; Pierce, L.E.; Sharik, T.L.; Bergen, K.M.; Kellndorfer, J.; Kendra, J.R.; Li, E.; Lin, Y.C.; Nashashibi, A.; et al. Estimation of forest biophysical characteristics in Northern Michigan with SIR-C/X-SAR. IEEE Trans. Geosci. Remote Sens. 1995, 33, 877–895. [Google Scholar] [CrossRef]
- Kasischke, E.S.; Smith, K.B.; Bourgeau-Chavez, L.L.; Romanowicz, E.A.; Brunzell, S.; Richardson, C.J. Effects of seasonal hydrologic patterns in south Florida wetlands on radar backscatter measured from ERS-2 SAR imagery. Remote Sens. Environ. 2003, 88, 423–441. [Google Scholar] [CrossRef]
- Pope, K.O.; Rejmankova, E.; Paris, J.F.; Woodruff, R. Detecting seasonal flooding cycles in marshes of the Yucatan Peninsula with SIR-C polarimetric radar imagery. Remote Sens. Environ. 1997, 58, 157–166. [Google Scholar] [CrossRef]
- Wang, Y.; Davis, F.W.; Melack, J.M.; Kasischke, E.S.; Christensen, N.L. The effects of changes in forest biomass on radar backscatter from tree canopies. Int. J. Remote Sens. 1995, 16, 503–513. [Google Scholar] [CrossRef]
- Plank, S.; Jüssi, M.; Martinis, S.; Twele, A. Mapping of flooded vegetation by means of polarimetric Sentinel-1 and ALOS-2/PALSAR-2 imagery. Int. J. Remote Sens. 2017, 38, 3831–3850. [Google Scholar] [CrossRef]
- Tsyganskaya, V.; Martinis, S.; Marzahn, P.; Ludwig, R.; Tsyganskaya, V.; Martinis, S.; Marzahn, P.; Ludwig, R. Detection of Temporary Flooded Vegetation Using Sentinel-1 Time Series Data. Remote Sens. 2018, 10, 1286. [Google Scholar] [CrossRef]
- Tsyganskaya, V.; Martinis, S.; Marzahn, P.; Ludwig, R. SAR-based detection of flooded vegetation—A review of characteristics and approaches. Int. J. Remote Sens. 2018, 39, 2255–2293. [Google Scholar] [CrossRef]
- Clarke, A.D.; Noone, K.J. Soot in the Arctic snowpack: A cause for perturbations in radiative transfer. Atmos. Environ. 1985, 19, 2045–2053. [Google Scholar] [CrossRef]
- Ettritch, G.; Hardy, A.; Bojang, L.; Cross, D.; Bunting, P.; Brewer, P. Enhancing digital surface models for hydraulic modelling using flood frequency detection. Remote Sens. Environ. 2018, 217, 506–522. [Google Scholar] [CrossRef]
- Gimnig, J.E.; Ombok, M.; Kamau, L.; Hawley, W.A. Characteristics of larval anopheline (Diptera: Culicidae) habitats in Western Kenya. J. Med. Entomol. 2001, 38, 282–288. [Google Scholar] [CrossRef]
- Fillinger, U.; Sonye, G.; Killeen, G.F.; Knols, B.G.J.; Becker, N. The practical importance of permanent and semipermanent habitats for controlling aquatic stages of Anopheles gambiae sensu lato mosquitoes: Operational observations from a rural town in western Kenya. Trop. Med. Int. Heal. 2004, 9, 1274–1289. [Google Scholar] [CrossRef]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). Publ. South. Afr. Inst. Med. Res. 1968, 54, 1–343. [Google Scholar]
- Fornadel, C.M.; Norris, L.C.; Franco, V.; Norris, D.E. Unexpected Anthropophily in the Potential Secondary Malaria Vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector-Borne Zoonotic Dis. 2011, 11, 1173–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, N.F.; Laurent, B. St.; Sikaala, C.H.; Hamainza, B.; Chanda, J.; Chinula, D.; Krishnankutty, S.M.; Mueller, J.D.; Deason, N.A.; Hoang, Q.T.; et al. Unexpected diversity of Anopheles species in Eastern Zambia: Implications for evaluating vector behavior and interventions using molecular tools. Sci. Rep. 2016, 5, 17952. [Google Scholar] [CrossRef]
- Amvongo-Adjia, N.; Wirsiy, E.L.; Riveron, J.M.; Chounna Ndongmo, W.P.; Enyong, P.A.; Njiokou, F.; Wondji, C.S.; Wanji, S. Bionomics and vectorial role of anophelines in wetlands along the volcanic chain of Cameroon. Parasites Vectors 2018, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Sexton, J.O.; Channan, S.; Townshend, J.R. A global, high-resolution (30-m) inland water body dataset for 2000: First results of a topographic–spectral classification algorithm. Int. J. Digit. Earth 2016, 9, 113–133. [Google Scholar] [CrossRef]
- Pekel, J.-F.; Cottam, A.; Gorelick, N.; Belward, A.S. High-resolution mapping of global surface water and its long-term changes. Nature 2016, 540, 418–422. [Google Scholar] [CrossRef]
- Klein, I.; Dietz, A.; Gessner, U.; Dech, S.; Kuenzer, C. Results of the Global WaterPack: A novel product to assess inland water body dynamics on a daily basis. Remote Sens. Lett. 2015, 6, 78–87. [Google Scholar] [CrossRef]
- Wendleder, A.; Wessel, B.; Roth, A.; Breunig, M.; Martin, K.; Wagenbrenner, S. TanDEM-X Water Indication Mask: Generation and First Evaluation Results. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Dargie, G.C.; Lewis, S.L.; Lawson, I.T.; Mitchard, E.T.A.; Page, S.E.; Bocko, Y.E.; Ifo, S.A. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 2017, 542, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Moore, A.E.; Cotterill, F.P.D.; Main, M.P.L.; Williams, H. The Zambezi River. In Large Rivers: Geomorphology and Management; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Zimba, H.; Kawawa, B.; Chabala, A.; Phiri, W.; Selsam, P.; Meinhardt, M.; Nyambe, I. Assessment of trends in inundation extent in the Barotse Floodplain, upper Zambezi River Basin: A remote sensing-based approach. J. Hydrol. Reg. Stud. 2018, 15, 149–170. [Google Scholar] [CrossRef]
- Kling, H.; Stanzel, P.; Preishuber, M. Impact modelling of water resources development and climate scenarios on Zambezi River discharge. J. Hydrol. Reg. Stud. 2014, 1, 17–43. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Haile, A.T.; Magidi, J.; Mapedza, E.; Nhamo, L. Living with floods—Household perception and satellite observations in the Barotse floodplain, Zambia. Phys. Chem. Earth, Parts A/B/C 2017, 100, 278–286. [Google Scholar] [CrossRef]
- Dunne, T.; Aalto, R. Large River Floodplains. In Treatise on Geomorphology; Shroder, J., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 645–678. [Google Scholar]
- Latrubesse, E.M. Patterns of anabranching channels: The ultimate end-member adjustment of mega rivers. Geomorphology 2008, 101, 130–145. [Google Scholar] [CrossRef]
- IUCN. Barotse Floodplain, Zambia: Local Economic Dependence on Wetland Resources; IUCN: Harare, Zimbabwe, 2003. [Google Scholar]
- Day, G.; Dietrich, W.E.; Rowland, J.C.; Marshall, A. The depositional web on the floodplain of the Fly River, Papua New Guinea. J. Geophys. Res. 2008, 113, F01S02. [Google Scholar] [CrossRef]
- Mertes, L.A.K. Documentation and significance of the perirheic zone on inundated floodplains. Water Resour. Res. 1997, 33, 1749–1762. [Google Scholar] [CrossRef] [Green Version]
- Burrough, S.L.; Thomas, D.S.G.; Oriojemie, E.A.; Willis, K.J. Landscape sensitivity and ecological change in western Zambia: The long-term perspective from dambo cut-and-fill sediments. J. Quat. Sci. 2015, 30, 44–58. [Google Scholar] [CrossRef]
- Lewin, J.; Ashworth, P.J. Defining large river channel patterns: Alluvial exchange and plurality. Geomorphology 2014, 215, 83–98. [Google Scholar] [CrossRef] [Green Version]
- Zambia Ministry of Health. Zambia National Malaria Indicator Survey; Ministry of Health: Lusaka, Zambia, 2012.
- Del Rio, T.; Groot, J.C.J.; DeClerck, F.; Estrada-Carmona, N. Integrating local knowledge and remote sensing for eco-type classification map in the Barotse Floodplain, Zambia. Data Br. 2018, 19, 2297–2304. [Google Scholar] [CrossRef]
- Timberlake, J. Biodiversity of the Zambezi Basin; Biodiversity Foundation for Africa: Bulawayo, Zimbabwe, 2000. [Google Scholar]
- Esch, T.; Taubenböck, H.; Roth, A.; Heldens, W.; Felbier, A.; Thiel, M.; Schmidt, M.; Müller, A.; Dech, S. TanDEM-X mission—New perspectives for the inventory and monitoring of global settlement patterns. J. Appl. Remote Sens. 2012, 6, 061702. [Google Scholar] [CrossRef]
- Bunting, P.; Clewley, D.; Lucas, R.M.; Gillingham, S. The Remote Sensing and GIS Software Library (RSGISLib). Comput. Geosci. 2014, 62, 216–226. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Kwak, Y.; Yun, S.; Iwami, Y. A new approach for rapid urban flood mapping using ALOS-2/PALSAR-2 in 2015 Kinu River Flood, Japan. In Proceedings of the 2017 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Fort Worth, TX, USA, 23–28 July 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1880–1883. [Google Scholar]
- Lopes, A.; Touzi, R.; Nezry, E. Adaptive speckle filters and scene heterogeneity. IEEE Trans. Geosci. Remote Sens. 1990, 28, 992–1000. [Google Scholar] [CrossRef]
- Clewley, D.; Bunting, P.; Shepherd, J.; Gillingham, S.; Flood, N.; Dymond, J.; Lucas, R.; Armston, J.; Moghaddam, M. A Python-Based Open Source System for Geographic Object-Based Image Analysis (GEOBIA) Utilizing Raster Attribute Tables. Remote Sens. 2014, 6, 6111–6135. [Google Scholar] [CrossRef] [Green Version]
- Rennó, C.D.; Nobre, A.D.; Cuartas, L.A.; Soares, J.V.; Hodnett, M.G.; Tomasella, J.; Waterloo, M.J. HAND, a new terrain descriptor using SRTM-DEM: Mapping terra-firme rainforest environments in Amazonia. Remote Sens. Environ. 2008, 112, 3469–3481. [Google Scholar] [CrossRef]
- Twele, A.; Cao, W.; Plank, S.; Martinis, S. Sentinel-1-based flood mapping: A fully automated processing chain. Int. J. Remote Sens. 2016, 37, 2990–3004. [Google Scholar] [CrossRef]
- Geurts, P.; Ernst, D.; Wehenkel, L. Extremely randomized trees. Mach. Learn. 2006, 63, 3–42. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, A.E.; Warner, T.A.; Fang, F. Implementation of machine-learning classification in remote sensing: An applied review. Int. J. Remote Sens. 2018, 39, 2784–2817. [Google Scholar] [CrossRef]
- Grenier, M.; Demers, A.-M.; Labrecque, S.; Benoit, M.; Fournier, R.A.; Drolet, B. An object-based method to map wetland using RADARSAT-1 and Landsat ETM images: Test case on two sites in Quebec, Canada. Can. J. Remote Sens. 2007, 33, S28–S45. [Google Scholar] [CrossRef]
- Harvey, K.R.; Hill, G.J.E. Vegetation mapping of a tropical freshwater swamp in the Northern Territory, Australia: A comparison of aerial photography, Landsat TM and SPOT satellite imagery. Int. J. Remote Sens. 2001, 22, 2911–2925. [Google Scholar] [CrossRef] [Green Version]
- Michishita, R.; Gong, P.; Xu, B. Spectral mixture analysis for bi-sensor wetland mapping using Landsat TM and Terra MODIS data. Int. J. Remote Sens. 2012, 33, 3373–3401. [Google Scholar] [CrossRef]
- Pontius, R.G.; Millones, M. Death to Kappa: Birth of quantity disagreement and allocation disagreement for accuracy assessment. Int. J. Remote Sens. 2011, 32, 4407–4429. [Google Scholar] [CrossRef]
- Warrens, M.J. Relative quantity and allocation disagreement measures for category-level accuracy assessment. Int. J. Remote Sens. 2015, 36, 5959–5969. [Google Scholar] [CrossRef]
- Comber, A.; Fisher, P.; Brunsdon, C.; Khmag, A. Spatial analysis of remote sensing image classification accuracy. Remote Sens. Environ. 2012, 127, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Haas, J.; Ban, Y. Urban growth and environmental impacts in Jing-Jin-Ji, the Yangtze, River Delta and the Pearl River Delta. Int. J. Appl. Earth Obs. Geoinf. 2014, 30, 42–55. [Google Scholar] [CrossRef]
- Bennett, N.D.; Croke, B.F.W.; Guariso, G.; Guillaume, J.H.A.; Hamilton, S.H.; Jakeman, A.J.; Marsili-Libelli, S.; Newham, L.T.H.; Norton, J.P.; Perrin, C.; et al. Characterising performance of environmental models. Environ. Model. Softw. 2013, 40, 1–20. [Google Scholar] [CrossRef]
- Olofsson, P.; Foody, G.M.; Stehman, S.V.; Woodcock, C.E. Making better use of accuracy data in land change studies: Estimating accuracy and area and quantifying uncertainty using stratified estimation. Remote Sens. Environ. 2013, 129, 122–131. [Google Scholar] [CrossRef]
- Olofsson, P.; Foody, G.M.; Herold, M.; Stehman, S.V.; Woodcock, C.E.; Wulder, M.A. Good practices for estimating area and assessing accuracy of land change. Remote Sens. Environ. 2014, 148, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.; Srivastava, H.S.; Navalgund, R.R. Use of synthetic aperture radar polarimetry to characterize wetland targets of Keoladeo National Park, Bharatpur, India. Curr. Sci. 2009, 97, 529–537. [Google Scholar]
- Thullen, J.S.; Sartoris, J.J.; Walton, W.E. Effects of vegetation management in constructed wetland treatment cells on water quality and mosquito production. Ecol. Eng. 2002, 18, 441–457. [Google Scholar] [CrossRef] [Green Version]
- Oesterholt, M.; Bousema, J.T.; Mwerinde, O.K.; Harris, C.; Lushino, P.; Masokoto, A.; Mwerinde, H.; Mosha, F.W.; Drakeley, C.J. Spatial and temporal variation in malaria transmission in a low endemicity area in northern Tanzania. Malar. J. 2006, 5, 98. [Google Scholar] [CrossRef]
- Fillinger, U.; Sombroek, H.; Majambere, S.; Van Loon, E.; Takken, W.; Lindsay, S.W. Identifying the most productive breeding sites for malaria mosquitoes in The Gambia. Malar. J. 2009, 8, 62. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasites Vectors 2010, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- McCrae, A. Oviposition by African malaria vector mosquitoes. II. Effects of site tone, water type and conspecific immatures on target selection by freshwater Anopheles gambiae. Ann. Trop. Med. Parasitol. 1984, 78, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Paaijmans, K.P.; Takken, W.; Githeko, A.K.; Jacobs, A.F.G. The effect of water turbidity on the near-surface water temperature of larval habitats of the malaria mosquito Anopheles gambiae. Int. J. Biometeorol. 2008, 52, 747–753. [Google Scholar] [CrossRef] [Green Version]















| Dataset | Dates | Area (UL, LR Coordinate WGS84) | Description |
|---|---|---|---|
| Classification datasets | |||
| Sentinel-1 | October 2016 to September 2018 (n = 59) | 22, −14; 24, −17 | Level-1 Ground Range Detected (GRD), VV and VH polarisation (10 m) |
| JRC Global Water Occurrence | Static | 22, −14; 24, −17 | Water body frequency derived from optical Landsat imagery (30 m) [56] |
| SRTM 3-second arc | Static | 22, −14; 24, −17 | Digital surface model (90 m) |
| Global Urban Footprint | Static | 22, −14; 24, −17 | Urban mask (12 m) derived from X-band TerraSAR-X imagery [74] |
| Validation datasets | |||
| Pleiades | July and March 2017 (n = 2) | 22.7, −15.6; 23.3, −15.1 | Visible-Near Infrared optical imagery (2 m) |
| Sentinel-2 | October 2016 to June 2018 (n = 11) | 22.8, −15.5; 23.6, −14.5 | Visible-Near Infrared optical imagery (10 m) |
| User’s Accuracy % | Producer’s Accuracy % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Num | Water | Other | VegWater | Water | Other | VegWater | Acc % | Kappa | ||
| Jan | 12/01/2018 | 903 | 98 | 82 | 99 | 99 | 96 | 85 | 92.7 | 0.89 |
| Feb | 05/02/2018 | 902 | 97 | 73 | 100 | 99 | 96 | 79 | 89.8 | 0.85 |
| Mar | 30/03/2017 | 903 | 100 | 74 | 100 | 100 | 100 | 80 | 91.4 | 0.87 |
| Apr | 18/04/2018 | 903 | 99 | 92 | 99 | 99 | 99 | 93 | 96.7 | 0.95 |
| May | 12/05/2018 | 903 | 95 | 69 | 99 | 99 | 92 | 77 | 87.8 | 0.82 |
| Jun | 17/06/2018 | 903 | 95 | 91 | 97 | 98 | 93 | 93 | 94.7 | 0.92 |
| Jul | 16/07/2017 | 903 | 99 | 89 | 97 | 99 | 96 | 91 | 95.2 | 0.93 |
| Aug | 09/08/2017 | 903 | 99 | 89 | 93 | 99 | 92 | 90 | 93.9 | 0.91 |
| Sep | 14/09/2017 | 903 | 98 | 88 | 93 | 99 | 91 | 90 | 93.1 | 0.9 |
| Oct | 13/10/2016 | 903 | 93 | 93 | 87 | 100 | 83 | 93 | 91.0 | 0.87 |
| Nov | 06/11/2016 | 851 | 95 | 89 | 69 | 99 | 71 | 88 | 84.4 | 0.77 |
| Dec | Cloudy | - | - | - | - | - | - | - | - | - |
| Mean | 97.1 | 84.6 | 94.1 | 99.1 | 91.6 | 87.2 | 91.9 | 0.9 | ||
| St Dev | 2.1 | 8.5 | 9.1 | 0.5 | 8.2 | 6.0 | 3.6 | 0.1 | ||
| Total points | 9880 | |||||||||
| Acc % | Kappa | Q | A | C | D | ||
|---|---|---|---|---|---|---|---|
| Jan | 12/01/2018 | 92.7 | 0.89 | 0.162 | 0.003 | 0.83 | 0.165 |
| Feb | 05/02/2018 | 89.8 | 0.85 | 0.241 | 0.001 | 0.75 | 0.245 |
| Mar | 30/03/2017 | 91.4 | 0.87 | 0.201 | 0.000 | 0.80 | 0.201 |
| Apr | 18/04/2018 | 96.7 | 0.95 | 0.058 | 0.003 | 0.94 | 0.062 |
| May | 12/05/2018 | 87.8 | 0.82 | 0.253 | 0.008 | 0.74 | 0.261 |
| Jun | 17/06/2018 | 94.7 | 0.92 | 0.084 | 0.002 | 0.91 | 0.085 |
| Jul | 16/07/2017 | 95.2 | 0.93 | 0.108 | 0.001 | 0.89 | 0.108 |
| Aug | 09/08/2017 | 93.9 | 0.91 | 0.104 | 0.001 | 0.89 | 0.105 |
| Sep | 14/09/2017 | 93.1 | 0.9 | 0.115 | 0.001 | 0.88 | 0.116 |
| Oct | 13/10/2016 | 91.0 | 0.87 | 0.044 | 0.004 | 0.95 | 0.048 |
| Nov | 06/11/2016 | 84.4 | 0.77 | 0.097 | 0.012 | 0.89 | 0.108 |
| Dec | Cloudy | - | - | - | - | - | - |
| Mean | 91.9 | 0.88 | 0.133 | 0.003 | 0.86 | 0.137 | |
| St Dev | 3.6 | 0.05 | 0.071 | 0.004 | 0.07 | 0.072 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardy, A.; Ettritch, G.; Cross, D.E.; Bunting, P.; Liywalii, F.; Sakala, J.; Silumesii, A.; Singini, D.; Smith, M.; Willis, T.; et al. Automatic Detection of Open and Vegetated Water Bodies Using Sentinel 1 to Map African Malaria Vector Mosquito Breeding Habitats. Remote Sens. 2019, 11, 593. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050593
Hardy A, Ettritch G, Cross DE, Bunting P, Liywalii F, Sakala J, Silumesii A, Singini D, Smith M, Willis T, et al. Automatic Detection of Open and Vegetated Water Bodies Using Sentinel 1 to Map African Malaria Vector Mosquito Breeding Habitats. Remote Sensing. 2019; 11(5):593. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050593
Chicago/Turabian StyleHardy, Andy, Georgina Ettritch, Dónall E. Cross, Pete Bunting, Francis Liywalii, Jacob Sakala, Andrew Silumesii, Douglas Singini, Mark Smith, Tom Willis, and et al. 2019. "Automatic Detection of Open and Vegetated Water Bodies Using Sentinel 1 to Map African Malaria Vector Mosquito Breeding Habitats" Remote Sensing 11, no. 5: 593. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050593