OCO-2 Solar-Induced Chlorophyll Fluorescence Variability across Ecoregions of the Amazon Basin and the Extreme Drought Effects of El Niño (2015–2016)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Datasets and Analysis
2.2.1. Solar-Induced Fluorescence from OCO-2
2.2.2. Forest Cover Data
2.2.3. Drought Indicator
2.2.4. Temperature and Vapor Pressure Deficit (VPD)
2.2.5. Absorbed Fraction of the Photosynthetically Active Radiation (fPAR)
2.2.6. Determining the Beginning of the Wet Season
2.3. Evaluation of SIF757 Patterns
3. Results
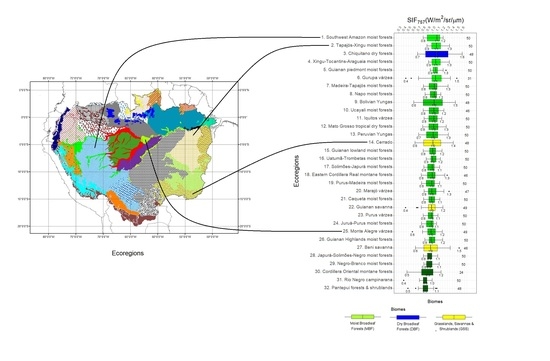
3.1. SIF757 Phenological Diversity across ER and Climate Zones
3.2. SIF757 and fPAR Dynamics at Climate Scale
3.3. Drought Extent and Development
3.4. SIF757 Response during the 2015/2016 El Niño Drought Event
4. Discussion
4.1. Phenological Diversity of SIF757
4.2. SIF757 and fPAR Dynamics at the Scale of Climate Zones
4.3. Drought Definition for Assessing SIF Variation during Drought
4.4. SIF757 Response during the El Niño 2015–2016 Extreme Drought Event
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Biome | ER | Köppen–Geiger Climate Zone(s) |
|---|---|---|
| Moist Broadleaf Forests (MBFs) | Bolivian Yungas | Af - Am - Aw - BWh - BSk - Cfc - Cfa - Cfb - Cwb - Cwc - ET |
| Caqueta moist forests | Af - Am | |
| Cordillera Oriental montane forests | Af - Am - Cfb | |
| Eastern Cordillera Real montane forests | Af - Am - Aw - BWh - As - Cfc - Cfb - Cwb - ET | |
| Guianan Highland moist forests | Af - Am - Aw - Cfb | |
| Guianan lowland moist forests | Af - Am - Aw | |
| Guianan piedmont moist forests | Af - Am - Aw - Cfb | |
| Gurupa várzea | Am - Aw | |
| Iquitos várzea | Af - Am - Aw | |
| Japurá–Solimões–Negro moist forests | Af | |
| Juruá–Purus moist forests | Af - Am - Aw | |
| Madeira–Tapajós moist forests | Af - Am - Aw | |
| Magdalena Valley montane forests | Af - Cfb | |
| Marajó várzea | Af - Am - Aw | |
| Marañón dry forests | Af - Am - Aw - BWh - BSk - Cfb - Cwb - Cwc - ET | |
| Mato Grosso tropical dry forests | Am - Aw | |
| Monte Alegre várzea | Af - Am - Aw | |
| Napo moist forests | Af - Am - As | |
| Negro–Branco moist forests | Af - Am - Cfb | |
| Northwest Andean montane forests | Cfc - Cfb - ET | |
| Pantepui forests and shrublands | Af - Am - Cfb | |
| Peruvian Yungas | Af - Am - Aw - BWh - BSk - Cfc - Cfb - Cwc - Cwb - ET | |
| Purus–Madeira moist forests | Af - Am - Aw | |
| Purus várzea | Af - Am - Aw | |
| Rio Negro campinarana | Af - Am | |
| Solimões–Japurá moist forests | Af | |
| Southern Andean Yungas | Aw - BWh - BSk - Cfa- Cfb - Cwa - Cwb | |
| Southwest Amazon moist forests | Af - Am - Aw - BWh - Cfb | |
| Tapajós–Xingu moist forests | Am - Aw | |
| Uatumã–Trombetas moist forests | Af - Am - Aw | |
| Ucayali moist forests | Af - Am - Aw - Cfb | |
| Xingu-Tocantins-Araguaia moist forests | Af - Am - Aw | |
| Dry Broadleaf Forests (DBFs) | Beni savanna | Af - Am - Aw |
| Bolivian montane dry forests | Aw - BWh - BSk - ET - Cfb - Cwb | |
| Chiquitano dry forests | Am - Aw | |
| Magdalena Valley dry forests | Af - Cfb | |
| Tumbes–Piura dry forests | Cfb - Cwb - ET | |
| Grasslands, Savannas and Shrublands (GSSs) | Central Andean dry puna | BSk - Cwc - Cwb - ET |
| Cerrado | Aw | |
| Dry Chaco | BWh - Aw | |
| Guianan savanna | Af - Am - Aw | |
| Montane Grasslands and Shrublands (MGSs) | Central Andean puna | BSk - BWh - Cfb - Cfc - Cwc - Cwb - ET |
| Central Andean wet puna | ET - Cfb - Cwc - Cwb | |
| Cordillera Central páramo | Af - Am - Aw - BSk - Cfc - Cfb - Cwc - Cwb - ET | |
| Northern Andean páramo | Af - Cfc - Cfb - ET | |
| Flooded Grasslands and Savannas (FGSs) | Pantanal | Aw |
| Deserts and Xeric Shrublands (DXSs) | Sechura desert | ET |
| Ecoregion | Quantity of SIF Samples (‰ over Total SIF Retrievals)/Mean SIF Value (W/m2/sr/um) | % of Change in SIF in Respect to Non-Drought | |||
|---|---|---|---|---|---|
| Ecoregional Area (km2) within the Amazon Basin | Drought Classes | Moderate/Severe and Extreme-Drought Areas | |||
| Non-Drought | Moderate | Severe/Extreme | |||
| 1. Southwest Amazon moist forests | 746,572 | 106.62/1.09 | 0.36/1.15 | -/- | 5.98/- |
| 2. Tapajós–Xingu moist forests | 335,000 | 40.99/1.15 | 5.87/1.09 | 9.09/1.00 | −5.45/−13.43 |
| 4. Xingu-Tocantins–Araguaia moist forests | 165,107 | 12.15/1.23 | 1.08/0.94 | 4.83/0.96 | −23.64/−22.15 |
| 5. Guianan piedmont moist forests | 88,536 | 29.8/1.04 | 3.06/1.08 | 24.85/1.08 | 4.45/4.45 |
| 6. Gurupa várzea | 10,170 | 0.31/1.05 | 0.22/1.12 | 0.06/1.29 | 6.93/23.39 |
| 7. Madeira–Tapajós moist forests | 716,661 | 78.25/1.09 | 1.87/0.75 | 1.7/0.83 | −31.09/−23.90 |
| 8. Napo moist forests | 250,652 | 64.57/1.09 | 1.02/0.98 | -/- | −10.29/- |
| 9. Bolivian Yungas | 90,330 | 4.83/1.23 | 0.12/1.18 | -/- | −4.54/- |
| 10. Ucayali moist forests | 114,259 | 6.16/1.12 | 0.01/1.57 | -/- | 39.76/- |
| 11. Iquitos várzea | 114,259 | 16.81/1.08 | -/- | -/- | -/- |
| 12. Mato Grosso tropical dry forests | 306,884 | 8.06/1.10 | 0.92/1.15 | 1.97/1.03 | 4.83/−6.36 |
| 13. Peruvian Yungas | 175,875 | 2.29/1.00 | -/- | -/- | -/- |
| 15. Guianan lowland moist forests | 34,696 | 12.54/1.09 | 18.66/1.02 | 4.93/0.97 | −6.47/−10.48 |
| 16. Uatumã–Trombetas moist forests | 444,474 | 104.98/1.01 | 68.95/1.01 | 109.99/1.00 | −0.73/−1.66 |
| 17. Solimões–Japurá moist forests | 166,902 | 7.75/1.05 | -/- | -/- | -/- |
| 18. Eastern Cordillera Real montane forests | 81,955 | 13.69/1.08 | 0.18/1.18 | -/- | 9.58/- |
| 19. Purus–Madeira moist forests | 173,482 | 39.43/1.01 | 0.37/0.99 | 2.01/0.99 | −1.98/−2.15 |
| 20. Marajó várzea | 40,080 | 0.87/1.00 | 0.54/1.04 | 4.07/0.95 | 4.12/−4.67 |
| 21. Caqueta moist forests | 168,098 | 0.08/1.02 | -/- | -/- | -/- |
| 23. Purus várzea | 176,473 | 12.13/0.98 | -/- | -/- | -/- |
| 24. Juruá–Purus moist forests | 241,679 | 19.19/1.02 | -/- | -/- | -/- |
| 25. Monte Alegre várzea | 66,402 | 7.03/1.06 | 0.53/0.87 | 1.05/0.96 | −18.21/−9.51 |
| 26. Guianan Highlands moist forests | 34,098 | 10.09/1.00 | 2.03/0.92 | 5.23/1.01 | −8.57/0.62 |
| 28. Japurá–Solimões-Negro moist forests | 267,402 | 52.73/0.99 | 3.83/0.95 | 6.49/1.00 | −3.97/1.27 |
| 29. Negro–Branco moist forests | 93,322 | 29.63/0.95 | 0.69/0.88 | 4.11/0.84 | −7.01/−11.25 |
| 31. Rio Negro campinarana | 80,759 | 15.46/0.87 | 1.33/0.77 | 3.59/0.81 | −11.39/−7.13 |
| 32. Pantepui forests and shrublands | 6580 | 4.01/0.84 | 0.41/0.99 | 3.52/0.91 | 17.56/8.16 |
| Total SIF retrievals in this experiment: 130,413 | |||||
References
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.A.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S.; et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, R.J. Origin and Evolution of Tropical Rain Forests; Wiley: Chichester, UK, 2000. [Google Scholar]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Muñoz, J.C.; Mattar, C.; Barichivich, J.; Santamaría-Artigas, A.; Takahashi, K.; Malhi, Y.; Sobrino, J.A.; van der Schrier, G. Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015–2016. Sci. Rep. 2016, 6, 33130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, S.L.; Brando, P.M.; Phillips, O.L.; van der Heijden, G.M.F.; Nepstad, D. The 2010 Amazon drought. Science 2011, 331, 554. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.L.; Aragão, L.E.O.C.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-González, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A.; et al. Drought sensitivity of the Amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, G.; van Schaik, E.; Araújo, A.C.; Boersma, K.F.; Gärtner, A.; Killaars, L.; Kooreman, M.L.; Kruijt, B.; van der Laan-Luijkx, I.T.; von Randow, C.; et al. Widespread reduction in sun-induced fluorescence from the Amazon during the 2015/2016 El Niño. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170408. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Phillips, O.L.; Feldpausch, T.R.; Gloor, E.; Baker, T.R.; Lloyd, J.; Lopez-Gonzalez, G.; Monteagudo-Mendoza, A.; Malhi, Y.; Lewis, S.L.; et al. Long-term decline of the Amazon carbon sink. Nature 2015, 519, 344. [Google Scholar] [CrossRef] [Green Version]
- Frankenberg, C.; Berry, J. Solar induced chlorophyll fluorescence: Origins, relation to photosynthesis and retrieval. In Comprehensive Remote Sensing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 143–162. [Google Scholar]
- Parazoo, N.C.; Bowman, K.; Fisher, J.B.; Frankenberg, C.; Jones, D.B.A.; Cescatti, A.; Pérez-Priego, O.; Wohlfahrt, G.; Montagnani, L. Terrestrial gross primary production inferred from satellite fluorescence and vegetation models. Glob. Chang. Biol. 2014, 20, 3103–3121. [Google Scholar] [CrossRef]
- MacBean, N.; Maignan, F.; Bacour, C.; Lewis, P.; Peylin, P.; Guanter, L.; Köhler, P.; Gómez-Dans, J.; Disney, M. Strong constraint on modelled global carbon uptake using solar-induced chlorophyll fluorescence data. Sci. Rep. 2018, 8, 1973. [Google Scholar] [CrossRef]
- Bertani, G.; Wagner, F.; Anderson, L.; Aragão, L. Chlorophyll fluorescence data reveals climate-related photosynthesis seasonality in Amazonian forests. Remote Sens. 2017, 9, 1275. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Saatchi, S.S.; Yang, Y.; Myneni, R.B.; Frankenberg, C.; Chowdhury, D.; Bi, J. Satellite observation of tropical forest seasonality: Spatial patterns of carbon exchange in Amazonia. Environ. Res. Lett. 2015, 10, 84005. [Google Scholar] [CrossRef]
- Li, X.; Xiao, J.; He, B. Chlorophyll fluorescence observed by OCO-2 is strongly related to gross primary productivity estimated from flux towers in temperate forests. Remote Sens. Environ. 2018, 204, 659–671. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hu, Y.-G.; Ren, C.-Z.; Guo, L.-C.; Wang, C.-L.; Jiang, Y.; Wang, X.-J.; Phendukani, H.; Zeng, Z.-H. Effects of nitrogen application on chlorophyll fluorescence parameters and leaf gas exchange in naked oat. J. Integr. Agric. 2013, 12, 2164–2171. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Liu, H.; Hua, L.; Luo, Q.; Lin, Y.; He, P.; Feng, S.; Liu, J.; Ye, Q. Differential responses of stomata and photosynthesis to elevated temperature in two co-occurring subtropical forest tree species. Front. Plant Sci. 2018, 9, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coops, N.C.; Hilker, T.; Hall, F.G.; Nichol, C.J.; Drolet, G.G. Estimation of light-use efficiency of terrestrial ecosystems from space: A status report. Bioscience 2010, 60, 788–797. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Fu, R.; Dickinson, R.; Joiner, J.; Frankenberg, C.; Gu, L.; Xia, Y.; Fernando, N. Drought onset mechanisms revealed by satellite solar-induced chlorophyll fluorescence: Insights from two contrasting extreme events. J. Geophys. Res. 2015, 120, 2427–2440. [Google Scholar] [CrossRef]
- Sun, Y.; Frankenberg, C.; Wood, J.D.; Schimel, D.S.; Jung, M.; Guanter, L.; Drewry, D.T.; Verma, M.; Porcar-Castell, A.; Griffis, T.J.; et al. OCO-2 advances photosynthesis observation from space via solar-induced chlorophyll fluorescence. Science 2017, 358. [Google Scholar] [CrossRef] [Green Version]
- Keenan, T.; Sabate, S.; Gracia, C. The importance of mesophyll conductance in regulating forest ecosystem productivity during drought periods. Glob. Chang. Biol. 2010, 16, 1019–1034. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Mencuccini, M.; Minunno, F.; Salmon, Y.; Martínez-Vilalta, J.; Hölttä, T. Coordination of physiological traits involved in drought-induced mortality of woody plants. New Phytol. 2015, 208, 396–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilton, M.C.; Metz, J.; Tielbörger, K. Climatic niche groups: A novel application of a common assumption predicting plant community response to climate change. Perspect. Plant Ecol. Evol. Syst. 2016, 19, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Gitlin, A.R.; Sthultz, C.M.; Bowker, M.A.; Stumpf, S.; Paxton, K.L.; Kennedy, K.; Muñoz, A.; Bailey, J.K.; Whitham, T.G. Mortality gradients within and among dominant plant populations as barometers of ecosystem change during extreme drought. Conserv. Biol. 2006, 20, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.S.; Vargas, G.G.; Brodribb, T.J.; Schwartz, N.B.; Perez-Aviles, D.; Smith-Martin, C.M.; Becknell, J.M.; Aureli, F.; Blanco, R.; Calderón-Morales, E.; et al. A catastrophic tropical drought kills hydraulically vulnerable tree species. Glob. Chang. Biol. 2020. [Google Scholar] [CrossRef]
- Umaña, M.N.; Forero-Montaña, J.; Nytch, C.J.; Thompson, J.; Uriarte, M.; Zimmerman, J.; Swenson, N.G. Dry conditions and disturbance promote liana seedling survival and abundance. Ecology 2019, 100, e02556. [Google Scholar] [CrossRef] [Green Version]
- Guanter, L.; Frankenberg, C.; Dudhia, A.; Lewis, P.E.; Gómez-Dans, J.; Kuze, A.; Suto, H.; Grainger, R.G. Retrieval and global assessment of terrestrial chlorophyll fluorescence from GOSAT space measurements. Remote Sens. Environ. 2012, 121, 236–251. [Google Scholar] [CrossRef]
- Lee, J.-E.; Frankenberg, C.; van der Tol, C.; Berry, J.A.; Guanter, L.; Boyce, C.K.; Fisher, J.B.; Morrow, E.; Worden, J.R.; Asefi, S.; et al. Forest productivity and water stress in Amazonia: Observations from GOSAT chlorophyll fluorescence. Proc. Biol. Sci. 2013, 280, 20130171. [Google Scholar] [CrossRef] [Green Version]
- Merrick, T.; Pau, S.; Jorge, M.L.S.P.; Bennartz, R.; Silva, T.S.F. Spatiotemporal patterns and phenology of tropical vegetation solar-induced chlorophyll fluorescence across Brazilian biomes using satellite observations. Remote Sens. 2019, 11, 1746. [Google Scholar] [CrossRef] [Green Version]
- Wunch, D.; Wennberg, P.O.; Toon, G.C.; Connor, B.J.; Fisher, B.; Osterman, G.B.; Frankenberg, C.; Mandrake, L.; O’Dell, C.; Ahonen, P.; et al. A method for evaluating bias in global measurements of CO2 total columns from space. Atmos. Chem. Phys. 2011, 11, 12317–12337. [Google Scholar] [CrossRef] [Green Version]
- Wunch, D.; Toon, G.C.; Blavier, J.-F.L.; Washenfelder, R.A.; Notholt, J.; Connor, B.J.; Griffith, D.W.T.; Sherlock, V.; Wennberg, P.O. The total carbon column observing network. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 2087–2112. [Google Scholar] [CrossRef] [Green Version]
- O’Dell, C.W.; Connor, B.; Bösch, H.; Brien, D.; Frankenberg, C.; Castano, R.; Christi, M.; Eldering, D.; Fisher, B.; Gunson, M.; et al. The ACOS CO2 retrieval algorithm—Part 1: Description and validation against synthetic observations. Atmos. Meas. Tech. 2012, 5, 99–121. [Google Scholar]
- Crisp, D.; Fisher, B.M.; O’Dell, C.; Frankenberg, C.; Basilio, R.; Bösch, H.; Brown, L.R.; Castano, R.; Connor, B.; Deutscher, N.M.; et al. The ACOS CO2 retrieval algorithm—Part II: Global X CO2 data characterization. Atmos. Meas. Tech. 2012, 5, 687–707. [Google Scholar] [CrossRef] [Green Version]
- Frankenberg, C.; Pollock, R.; Lee, R.A.M.; Rosenberg, R.; Blavier, J.-F.; Crisp, D.; O’Dell, C.W.; Osterman, G.B.; Roehl, C.; Wennberg, P.O.; et al. The orbiting carbon observatory (OCO-2): Spectrometer performance evaluation using pre-launch direct sun measurements. Atmos. Meas. Tech. Discuss. 2014, 7, 7641–7670. [Google Scholar] [CrossRef]
- Frankenberg, C.; O’Dell, C.; Berry, J.; Guanter, L.; Joiner, J. Prospects for chlorophyll fluorescence remote sensing from the orbiting carbon observatory-2. Remote Sens. Environ. 2014, 147, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S.; Chen, J. Prediction of Satellite-based Column CO2 Concentration by Combining Emission Inventory and LULC information. IEEE Trans. Geosci. Remote Sens. 2020. [Google Scholar] [CrossRef]
- Köhler, P.; Frankenberg, C.; Magney, T.S. Global retrievals of solar-induced chlorophyll fluorescence with TROPOMI: First results and intersensor comparison to OCO-2. AGU Adv. Earth Space Sci. 2018. [Google Scholar] [CrossRef] [Green Version]
- Frankenberg, C.; Fisher, J.B.; Worden, J.; Badgley, G.; Saatchi, S.S.; Lee, J.-E.; Toon, G.C.; Butz, A.; Jung, M.; Kuze, A.; et al. New global observations of the terrestrial carbon cycle from GOSAT: Patterns of plant fluorescence with gross primary productivity. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef] [Green Version]
- Joiner, J.; Guanter, L.; Lindstrot, R.; Voigt, M.; Vasilkov, A.P.; Middleton, E.M.; Huemmrich, K.F.; Yoshida, Y.; Frankenberg, C. Global monitoring of terrestrial chlorophyll fluorescence from moderate-spectral-resolution near-infrared satellite measurements: Methodology, simulations, and application to GOME-2. Atmos. Meas. Tech. 2013, 6, 2803–2823. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Frankenberg, C.; Jung, M.; Joiner, J.; Guanter, L.; Köhler, P.; Magney, T. Overview of Solar-Induced chlorophyll Fluorescence (SIF) from the Orbiting Carbon Observatory-2: Retrieval, cross-mission comparison, and global monitoring for GPP. Remote Sens. Environ. 2018, 209, 808–823. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on earth. Bioscience 2001, 51, 933. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.M.; Graham, D.J.; Webster, A.L.; Primm, S.A.; Bookbinder, M.P.; Ledec, G. A Conservation Assessment of the Terrestrial Ecoregions of Latin America and the Caribbean; World Bank: Washington, DC, USA, 1995. [Google Scholar]
- Li, X.; Xiao, J.; He, B.; Altaf Arain, M.; Beringer, J.; Desai, A.R.; Emmel, C.; Hollinger, D.Y.; Krasnova, A.; Mammarella, I.; et al. Solar-induced chlorophyll fluorescence is strongly correlated with terrestrial photosynthesis for a wide variety of biomes: First global analysis based on OCO-2 and flux tower observations. Glob. Chang. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, E.; Logsdon, M.G.; Ballester, M.V.R.; Richey, J.E. LBA-ECO CD-06 Amazon river basin land and stream drainage direction maps. ORNL DAAC 2012. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. METZ 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Frankenberg, C. Solar Induced Chlorophyll Fluorescence: OCO-2 Lite Files (B7000) User Guide. Available online: https://docserver.gesdisc.eosdis.nasa.gov/public/project/OCO/OCO2_SIF_B7000_Product_Description_090215.pdf (accessed on 14 January 2020).
- NASA. OCO2 Data Portal. Available online: https://co2.jpl.nasa.gov/#mission=OCO-2 (accessed on 3 October 2019).
- Eldering, A.; Wennberg, P.O.; Crisp, D.; Schimel, D.S.; Gunson, M.R.; Chatterjee, A.; Liu, J.; Schwandner, F.M.; Sun, Y.; O’Dell, C.W.; et al. The orbiting carbon observatory-2 early science investigations of regional carbon dioxide fluxes. Science 2017, 358. [Google Scholar] [CrossRef] [Green Version]
- Goulas, Y.; Daumard, F.; Ounis, A.; Rhoul, C.; Lopez, M.L.; Moya, I. Monitoring the diurnal time course of vegetation dynamics with geostationary observations: The GFLEX project. In Proceedings of the 6th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Lausanne, Switzerland, 24–27 June 2014; pp. 1–4. [Google Scholar]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [Green Version]
- Wells, N.; Goddard, S.; Hayes, M.J. A self-calibrating palmer drought severity index. J. Clim. 2004, 17, 2335–2351. [Google Scholar] [CrossRef]
- Dai, A.; Trenberth, K.E.; Qian, T. A global dataset of palmer drought severity index for 1870–2002: Relationship with soil moisture and effects of surface warming. J. Hydrometeorol. 2004, 5, 1117–1130. [Google Scholar] [CrossRef]
- Van der Schrier, G.; Barichivich, J.; Briffa, K.R.; Jones, P.D. A scPDSI-based global data set of dry and wet spells for 1901–2009. J. Geophys. Res. Atmos. 2013, 118, 4025–4048. [Google Scholar] [CrossRef]
- Zang, C.S.; Buras, A.; Esquivel-Muelbert, A.; Jump, A.S.; Rigling, A.; Rammig, A. Standardized drought indices in ecological research: Why one size does not fit all. Glob. Chang. Biol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Dee, D.P.; Uppala, S.M.; Simmons, A.J.; Berrisford, P.; Poli, P.; Kobayashi, S.; Andrae, U.; Balmaseda, M.A.; Balsamo, G.; Bauer, P.; et al. The ERA-Interim reanalysis: Configuration and performance of the data assimilation system. Q. J. R. Meteorol. Soc. 2011, 137, 553–597. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Digital Soil Map of the World and Derived Soil Properties; FAO: Rome, Italy, 2003. [Google Scholar]
- Şen, Z. Applied Drought Modeling, Prediction, and Mitigation; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Yan, K.; Park, T.; Yan, G.; Chen, C.; Yang, B.; Liu, Z.; Nemani, R.; Knyazikhin, Y.; Myneni, R. Evaluation of MODIS LAI/FPAR product collection 6. Part 1: Consistency and improvements. Remote Sens. 2016, 8, 359. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Park, T.; Yan, G.; Liu, Z.; Yang, B.; Chen, C.; Nemani, R.; Knyazikhin, Y.; Myneni, R. Evaluation of MODIS LAI/FPAR product collection 6. Part 2: Validation and intercomparison. Remote Sens. 2016, 8, 460. [Google Scholar] [CrossRef] [Green Version]
- Huffman, G.J.; Bolvin, D.T.; Nelkin, E.J.; Wolff, D.B.; Adler, R.F.; Gu, G.; Hong, Y.; Bowman, K.P.; Stocker, E.F. The TRMM multisatellite precipitation analysis (TMPA): Quasi-global, multiyear, combined-sensor precipitation estimates at fine scales. J. Hydrometeor. 2007, 8, 38–55. [Google Scholar] [CrossRef]
- Wu, J.; Albert, L.P.; Lopes, A.P.; Restrepo-Coupe, N.; Hayek, M.; Wiedemann, K.T.; Guan, K.; Stark, S.C.; Christoffersen, B.; Prohaska, N.; et al. Leaf development and demography explain photosynthetic seasonality in Amazon evergreen forests. Science 2016, 351, 972–976. [Google Scholar] [CrossRef] [Green Version]
- Van der Tol, C.; Rossini, M.; Cogliati, S.; Verhoef, W.; Colombo, R.; Rascher, U.; Mohammed, G. A model and measurement comparison of diurnal cycles of sun-induced chlorophyll fluorescence of crops. Remote Sens. Environ. 2016, 186, 663–677. [Google Scholar] [CrossRef]
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy near-infrared reflectance and terrestrial photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef] [Green Version]
- Hans, H.; Dee, D.J.E.N. ERA5 reanalysis is in production. ECMWF Newsl. 2016, 147, 5–6. [Google Scholar]
- Dijkshoorn, J.A.; van Engelen, V.W.P.; Huting, J.R.M.; Tempel, P. Soil and Terrain Database for Latin America and the Caribbean (Version 2.0)—Scale 1:5 Million (SOTERLAC), [CD-ROM]; ISRIC, World Soil Information: Wageningen, The Netherlands, 2014. [Google Scholar]
- Lorenz, C.; Kunstmann, H. The hydrological cycle in three state-of-the-art reanalyses: Intercomparison and performance analysis. J. Hydrometeor. 2012, 13, 1397–1420. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M. Effects of water deficits on carbon assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Kitao, M.; Lei, T.T.; Koike, T.; Tobita, H.; Maruyama, Y.; Matsumoto, Y.; Ang, L.-H. Temperature response and photoinhibition investigated by chlorophyll fluorescence measurements for four distinct species of dipterocarp trees. Physiol. Plant. 2000, 109, 284–290. [Google Scholar] [CrossRef]
- Woo, N.S.; Badger, M.R.; Pogson, B.J. A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods 2008, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.O.; Galbraith, D.; Gloor, M.; Deurwaerder, H.; de Guimberteau, M.; Rammig, A.; Thonicke, K.; Verbeeck, H.; Randow, C.; von Monteagudo, A.; et al. Variation in stem mortality rates determines patterns of above-ground biomass in Amazonian forests: Implications for dynamic global vegetation models. Glob. Chang. Biol. 2016, 22, 3996–4013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Tian, H.; Pan, S.; Chen, G.; Zhang, B.; Dangal, S. Amazon drought and forest response: Largely reduced forest photosynthesis but slightly increased canopy greenness during the extreme drought of 2015/2016. Glob. Chang. Biol. 2018, 24, 1919–1934. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Schwalm, C.; Biondi, F.; Camarero, J.J.; Koch, G.; Litvak, M.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; Williams, A.P.; et al. FOREST ECOLOGY. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Anderegg, W.R.L.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef] [Green Version]
- Guadagno, C.R.; Ewers, B.E.; Speckman, H.N.; Aston, T.L.; Huhn, B.J.; DeVore, S.B.; Ladwig, J.T.; Strawn, R.N.; Weinig, C. Dead or alive? Using membrane failure and chlorophyll a fluorescence to predict plant mortality from drought. Plant Physiol. 2017, 175, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Gutbrodt, B.; Mody, K.; Dorn, S. Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos 2011, 120, 1732–1740. [Google Scholar] [CrossRef]
- Selwood, K.E.; Thomson, J.R.; Clarke, R.H.; McGeoch, M.A.; Mac Nally, R. Resistance and resilience of terrestrial birds in drying climates: Do floodplains provide drought refugia? Glob. Ecol. Biogeogr. 2015, 24, 838–848. [Google Scholar] [CrossRef]
- Matthews, W.J.; Marsh-Matthews, E. Effects of drought on fish across axes of space, time and ecological complexity. Freshw. Biol. 2003, 48, 1232–1253. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Medlyn, B.E.; Pitman, A.J.; Drake, J.E.; Ukkola, A.; Griebel, A.; Pendall, E.; Prober, S.; Roderick, M. Examining the evidence for decoupling between photosynthesis and transpiration during heat extremes. Biogeosciences 2019, 16, 903–916. [Google Scholar] [CrossRef] [Green Version]
- Paul-Limoges, E.; Damm, A.; Hueni, A.; Liebisch, F.; Eugster, W.; Schaepman, M.; Buchmann, N. Effect of environmental conditions on sun-induced fluorescence in a mixed forest and a cropland. Remote Sens. Environ. 2018, 219, 310–323. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: Mechanisms and challenges. J. Exp. Bot. 2014, 65, 4065–4095. [Google Scholar] [CrossRef] [PubMed]




| Dimension | Biomes | ER |
|---|---|---|
| Climatic | Experience comparable climatic regimes | Have similar environmental conditions |
| Biologic | Have similar vegetation structure | Share a large majority of their species |
| Ecologic | Display similar spatial patterns of biodiversity (e.g., levels of beta diversity) | Interact ecologically in ways that are critical for their long-term persistence |
| Contain flora and fauna with similar guild structures and life histories |
| Status | |||||
|---|---|---|---|---|---|
| Level | Dry | Non-Drought | |||
| scPDSI thresholds | ≤−4.0 | >−4.0, ≤−3.0 | >−3.0, ≤−2.0 | >−2.0, ≤−1.0 | >−1.0, <1.0 |
| P and PET thresholds | P – PET < 0 | P – PET ≥ 0 | |||
| Drought classification | Extreme | Severe | Moderate | Mild | Normal |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, A.O.; Chen, J.; Zang, C.S.; Shekhar, A.; Jimenez, J.C.; Bhattacharjee, S.; Kindu, M.; Morales, V.H.; Rammig, A. OCO-2 Solar-Induced Chlorophyll Fluorescence Variability across Ecoregions of the Amazon Basin and the Extreme Drought Effects of El Niño (2015–2016). Remote Sens. 2020, 12, 1202. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12071202
Castro AO, Chen J, Zang CS, Shekhar A, Jimenez JC, Bhattacharjee S, Kindu M, Morales VH, Rammig A. OCO-2 Solar-Induced Chlorophyll Fluorescence Variability across Ecoregions of the Amazon Basin and the Extreme Drought Effects of El Niño (2015–2016). Remote Sensing. 2020; 12(7):1202. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12071202
Chicago/Turabian StyleCastro, Antony Oswaldo, Jia Chen, Christian S. Zang, Ankit Shekhar, Juan Carlos Jimenez, Shrutilipi Bhattacharjee, Mengistie Kindu, Victor Hugo Morales, and Anja Rammig. 2020. "OCO-2 Solar-Induced Chlorophyll Fluorescence Variability across Ecoregions of the Amazon Basin and the Extreme Drought Effects of El Niño (2015–2016)" Remote Sensing 12, no. 7: 1202. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12071202