5.1. The Vineyard Crop
The NDVI, GNDVI, and SAVI indices maps were extracted from the processed orthoimage and are shown in
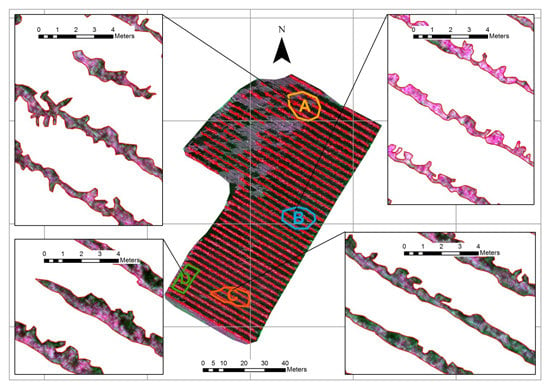
Figure 7. Within the spatial distributions of the NDVI, GNDVI, and SAVI values, the zones with negative behavior are well-recognizable (between orange and red). At the border zones of the vineyard, some red pixels often appear, representing negative values of the index, probably due to the gravel road surrounding the vineyard describing a situation similar to the bare soil. However, for all indices some low values can also be found inside the vineyard. On the other hand, some areas where the vegetation has good vigor attributes for all the indices are also discriminable in the field.
Figure 7.
The NDVI (a), GNDVI (b), and SAVI (c, with L = 0.25) maps for the vineyard area. Areas A–D and C–B show zones with high and low VI values, respectively.
Figure 7.
The NDVI (a), GNDVI (b), and SAVI (c, with L = 0.25) maps for the vineyard area. Areas A–D and C–B show zones with high and low VI values, respectively.
Concerning the NDVI index, the found value ranges between −0.45 and 1 (
Figure 7a) but only values ranging between 0.4 and 0.9 are compatible with the crop presence. In particular, the vineyard lines are recognized through the higher NDVI values.
Given that the reflectance in green regions is higher than in the red regions, for the GNDVI a different range with respect to the NDVI index was found. The GNDVI map has a maximum at 0.58 whereas the minimum is −0.33 (
Figure 7b). From a qualitative analysis, the areas with high NDVI values match to the areas with high GNDVI value.
For the SAVI computation (
Figure 7c), the characteristics of the surveyed vineyard area suggested choosing some low L values (L = 0.25 and L = 0.10). In both case, the resulting maps are similar.
As shown in
Figure 7b,c, the GNDVI and SAVI values exceed the traditional known ranges, probably due to:
- -
the presence of many shadows (dark areas) between the vineyard lines, which badly affected the result of these indices;
- -
the use of Digital Numbers instead of the corresponding reflectance values.
However, in all three maps it is possible to recognize the same spectral discrimination of the areas.
To confirm these qualitative considerations, a statistical analysis was performed using all vegetation index maps. Four areas of interest, named A, B, C, and D, were selected inside the vineyard and highlighted with different colors (
Figure 7). The white polygons indicate the large index value zones whereas the black polygons cover lower index value.
It is important to notice that the vineyard crop has a lack of plants in some areas, especially in the northwest zone (
Figure 8). In these areas, low VI values have been derived, again describing a situation similar to the bare soil.
Figure 8.
Map of the no-vine plant zones and the areas of interest used for the statistical analysis.
Figure 8.
Map of the no-vine plant zones and the areas of interest used for the statistical analysis.
Area A and Area D cover a region with better vegetation conditions, while Area B and Area C include pixels with lower NDVI values. For each area, basic statistics of indices values,
i.e., Mean (m) and Standard Deviation (σx), were calculated (
Table 5). In particular, the mean NDVI value reflects a mean productivity and biomass, whereas the standard deviation represents a measure of the spatial variability in productivity [
24]. The mean value classifications for all indices agree with the previous qualitative discrimination, confirming that the health condition of areas A and D is better than B and C.
Table 5.
Mean and Standard Deviation of sample areas A, B, C, and D.
Table 5.
Mean and Standard Deviation of sample areas A, B, C, and D.
| NDVI |
| Area | N° Pixel | Mean | Standard Deviation |
| A | 62,538 | 0.70 | 0.09 |
| B | 49,896 | 0.63 | 0.13 |
| C | 44,844 | 0.61 | 0.10 |
| D | 34,469 | 0.69 | 0.10 |
| GNDVI |
| Area | N° Pixel | Mean | Standard Deviation |
| A | 62,538 | 0.26 | 0.04 |
| B | 49,896 | 0.23 | 0.06 |
| C | 44,844 | 0.22 | 0.05 |
| D | 34,469 | 0.26 | 0.05 |
| SAVI |
| Area | N° Pixel | Mean | Standard Deviation |
| A | 62,538 | 0.87 | 0.12 |
| B | 49,896 | 0.78 | 0.16 |
| C | 44,844 | 0.76 | 0.13 |
| D | 34,469 | 0.86 | 0.13 |
However, as the previous statistic parameters are not able to fully explain the data distributions, in order to satisfy the needs of precision farming techniques, the relative frequency histogram of VI values were plotted for each area (
Figure 9). To avoid a histogram overlap and to provide a better understanding, two different graphs were drawn, one relating to areas A and B and the other relating to C and D. For all VI, the graphics show that the pixels belonging to areas A and B are distributed over higher VI values with respect to C and D, confirming again the previous findings.
As mentioned before, the high-resolution contents of the UAV images allow us to detect many details and features normally not visible in low-resolution aerial or satellite imagery. In the analyzed vineyard area, several covers are included besides grapevines,
i.e., ground vegetation, wood, shadows,
etc. Thus, to eliminate the spectral disturbances of these coverages, sub-areas were selected with a careful manual digitalization of the vineyard lines in order to separate the real cultivated areas from other parts (
Figure 10).
For each sub-area, basic statistics of VI values,
i.e., Mean (m) and Standard Deviation (σx), were calculated (
Table 6). In particular for area B, the mean VI value increase is approximately 13% for the NDVI, about 9% for the GNDVI and about 16% for the SAVI, while for other areas the increase never exceeds 7% (A and C areas always remain below 4%). These results indicate that actually in area B, the plants of the vineyard lines have a healthy state close to that of the plants in the best areas found previously (A and D). This consideration differs from the assessment made in the previous analysis, which referred to the whole areas of interest, demonstrating the advantage of using high-resolution images and selecting only areas with cultivation.
Figure 9.
Relative frequency histograms of NDVI, GNDVI, SAVI indices, and values for areas A, B, C, and D. Given the different nature of the GNDVI index, a different range was used for the horizontal axis of the graph.
Figure 9.
Relative frequency histograms of NDVI, GNDVI, SAVI indices, and values for areas A, B, C, and D. Given the different nature of the GNDVI index, a different range was used for the horizontal axis of the graph.
Finally, the relative frequency histograms of the sub-areas (
Figure 11) confirmed that the plants of Area C have a worse vegetative state than that of the vines included in the other areas of interest.
Figure 10.
The manually digitized vineyard lines for the areas under investigation (A, B, C, D) to compute the VI only inside the cultivated zones and eliminate possible spectral disturbs from ground vegetation, wood, shadows, etc.
Figure 10.
The manually digitized vineyard lines for the areas under investigation (A, B, C, D) to compute the VI only inside the cultivated zones and eliminate possible spectral disturbs from ground vegetation, wood, shadows, etc.
Table 6.
Mean and Standard Deviation of sample areas A, B, C, and D.
Table 6.
Mean and Standard Deviation of sample areas A, B, C, and D.
| NDVI |
| Area | N° Pixel | Mean | Standard Deviation |
| A | 11,750 | 0.72 | 0.10 |
| B | 7317 | 0.70 | 0.12 |
| C | 9399 | 0.65 | 0.10 |
| D | 9584 | 0.72 | 0.11 |
| GNDVI |
| Area | N° Pixel | Mean | Standard Deviation |
| A | 11,750 | 0.26 | 0.04 |
| B | 7317 | 0.25 | 0.05 |
| C | 9399 | 0.21 | 0.04 |
| D | 9584 | 0.25 | 0.05 |
| SAVI |
| Area | N° Pixel | Mean | Standard Deviation |
| A | 11,750 | 0.90 | 0.13 |
| B | 7317 | 0.87 | 0.16 |
| C | 9399 | 0.81 | 0.13 |
| D | 9584 | 0.89 | 0.14 |
Figure 11.
Relative frequency histograms of NDVI, GNDVI, SAVI, and values for areas A, B, C, and D. Given the different nature of the GNDVI index, a different range was used for the horizontal axis of the graph.
Figure 11.
Relative frequency histograms of NDVI, GNDVI, SAVI, and values for areas A, B, C, and D. Given the different nature of the GNDVI index, a different range was used for the horizontal axis of the graph.
From this analysis, it is possible to conclude that better health conditions are visible in the vineyard plants contained in areas A and D, while in B the vegetation state is slightly worse. Instead, the C area shows the more worrying situation. The local vineyard farmer confirmed that, being a biological culture, the vineyard is subject to attack by various pests/fungi, including especially
Armillaria mellea and the “Grapevine trunk diseases”. In fact, these are the cause of plant removal in the northwest area of the vineyard. The first is a plant pathogen that causes
Armillaria root rot in many plant species and produces mushrooms around the base of infected trees. The symptoms appear in the crowns of infected trees as discolored foliage, reduced growth, dieback of the branches, and death [
38]. Grapevine trunk diseases are a set of vine diseases caused by fungal species that colonize the lymph vessels and the wood, compromising the translocation of water and nutrients from the roots to the aerial part of the plant. The fungus produces toxins that cause the chlorotic and necrotic stains on the leaves. In grapes it is accompanied by the appearance of purplish spots on the berries [
39]. Both diseases are spread over the entire vineyard. However, since the trees that are already under stress are more likely to be attacked, the B area and in particular the C area are more vulnerable to these problems. In fact, these zones have a deficiency of fertilizer. The reason is a depression that has been backfilled in the past, but, despite having a similar composition to the rest of the ground below the vineyard, a lower intake of fertilizer was found.
Finally, the different behavior between area B and C is probably due to the irregular appearance of “Grapevine trunk diseases” symptoms from year to year. Often, the symptomatic plants during a season cannot manifest symptoms for several consecutive years, thus continuing to have the normal production of healthy plants.
5.2. The Tomato Crop
The NDVI, GNDVI, and SAVI indices were computed on the available orthoimage and the extracted VI maps are shown on
Figure 12. Compared to the vineyard, in herbaceous crops such as tomatoes the VI analysis is more complex. Indeed, between plants the growth is not uniform and the vegetation distribution on the ground is not homogeneous, with considerable presence of bare soil. As reported by the farm manager, the cause is the “Bacterial spot” presence strongly spread over the whole crop. Bacterial spot is one of the most devastating diseases of tomato fields due to the action of various bacteria that attack foliage, stems, and fruits. Symptoms begin as small, yellow-green lesions on young leaves, which usually appear deformed and twisted, or as dark lesions on older foliage. Lesions develop rapidly and the diseased leaves drop prematurely, resulting in extensive defoliation, while those leaves that remain on the plants may have a scorched appearance. Fruit spots begin as pale-green, water-soaked areas that eventually become raised, brown, and roughened on tomato fruit. Spots may provide entrance points for various fungal and other bacterial invaders that can cause secondary fruit rots [
40].
Within the spatial distributions of the NDVI, GNDVI, and SAVI values, the areas with negative behavior are easily recognizable (between orange and red). In the northwest and southeast border zones of the crop, some red pixels—representing negative index values—appear. The cause is probably the gravel road surrounding the field and leading to a situation similar to the bare soil. However, for all indices some low values can be found also inside the tomatoes’ area. Concerning the ranges of the computed VI, the same problem encountered for the vineyard occurs for the tomato crop, too. In particular, the GNDVI and SAVI values exceed the traditional known ranges, probably due to the use of Digital Numbers instead of the corresponding reflectance values.
Figure 12.
The NDVI, GNDVI, and SAVI (L = 0.5) maps for the tomato crop. Areas A and B show zones with high and low VI values, respectively.
Figure 12.
The NDVI, GNDVI, and SAVI (L = 0.5) maps for the tomato crop. Areas A and B show zones with high and low VI values, respectively.
Two sample areas (A and B) with different vegetation density were then selected. These areas were statistically analyzed, computing Mean (m) and Standard Deviation (σx) (
Table 7). The results suggest that the VI response is better for area A than B. In fact, for the former the average VI values are more than 30% Higher.
Table 7.
Mean and standard deviation of the sample areas A and B in the tomato crop.
Table 7.
Mean and standard deviation of the sample areas A and B in the tomato crop.
| NDVI |
| Area | N° Pixel | Mean | Standard Deviation |
| A | 107,638 | 0.59 | 0.18 |
| B | 107,819 | 0.45 | 0.23 |
| GNDVI |
| Area | N° Pixel | Mean | Standard Deviation |
| A | 107,638 | 0.22 | 0.08 |
| B | 107,819 | 0.17 | 0.10 |
| SAVI |
| Area | N° Pixel | Mean | Standard Deviation |
| A | 107,638 | 0.87 | 0.27 |
| B | 107,819 | 0.67 | 0.35 |
Moreover, considering the relative frequency histograms (
Figure 13), it is evident that a greater number of pixels in area B fall on lower values than in area A, indicating a worse health condition. However, it is necessary to emphasize that for tomato cultivation treatments are suspended some time before harvesting; this occurred in this case a few days after the UAV survey. Since, at this stage, some plants continue growth while others will dry on the soil, the decrease of the values of the indexes could not be linked to the state of health only.
Figure 13.
Relative frequency histograms of NDVI, GNDVI, and SAVI for areas A and B in the tomato crop.
Figure 13.
Relative frequency histograms of NDVI, GNDVI, and SAVI for areas A and B in the tomato crop.
Due to the uneven plant distribution on the ground, in the tomato crop case study we could not separate the individual plants through a manual digitization procedure.