Potentiometric NO2 Sensors Based on Thin Stabilized Zirconia Electrolytes and Asymmetric (La0.8Sr0.2)0.95MnO3 Electrodes
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Synthesis of Electrode Powders
2.2. Fabrication of Sensors

2.3. Evaluation of the Sensing Performances

3. Results and Discussion
3.1. Properties of LSM95 and Carbon Powders



3.2. Microstructure of Sensors



3.3. Influence of Porosity on Sensing Performance

3.4. Sensing Performance of Sensors with Different Sensing Materials



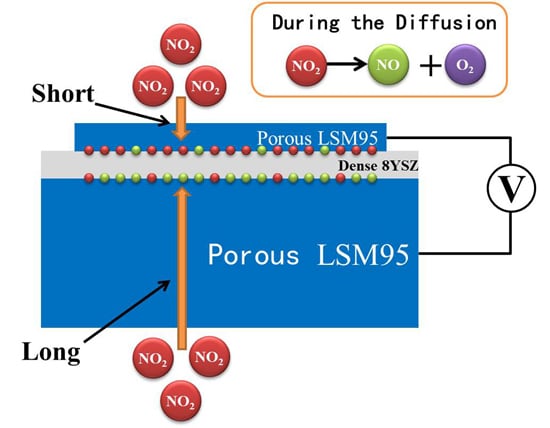
3.5. Sensing Mechanism

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mothe, G.; Castro, M.; Sthel, M.; Lima, G.; Brasil, L.; Campos, L.; Rocha, A.; Vargas, H. Detection of Greenhouse Gas Precursors from Diesel Engines Using Electrochemical and Photoacoustic Sensors. Sensors 2010, 10, 9726–9741. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Wu, J.; Liang, G.; Liu, J.; Chu, G.; Zhou, G.; Zhang, D. Effects of simulated acid rain on soil and soil solution chemistry in a monsoon evergreen broad-leaved forest in southern China. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Moos, R. Catalysts as Sensors—A Promising Novel Approach in Automotive Exhaust Gas Aftertreatment. Sensors 2010, 10, 6773–6787. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, X.; Dutta, P.K. Exploitation of Unique Properties of Zeolites in the Development of Gas Sensors. Sensors 2012, 12, 5170–5194. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef]
- Moos, R.; Sahner, K.; Fleischer, M.; Guth, U.; Barsan, N.; Weimar, U. Solid State Gas Sensor Research in Germany—A Status Report. Sensors 2009, 9, 4323–4365. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Miura, N.; Yamazoe, N. High-Temperature NO or NO2 Sensor Using Stabilized Zirconia and Tungsten Oxide Electrode. Ionics 1998, 4, 16–24. [Google Scholar] [CrossRef]
- Miura, N.; Akisada, K.; Wang, J.; Zhuiykov, S.; Ono, T. Mixed-potential-type NOx sensor based on YSZ and zinc oxide sensing electrode. Ionics 2004, 10, 1–9. [Google Scholar] [CrossRef]
- Miura, N.; Shiraishi, T.; Shimanoe, K.; Yamazoe, N. Mixed-potential-type propylene sensor based on stabilized zirconia and oxide electrode. Electrochem. Commun. 2000, 2, 77–80. [Google Scholar] [CrossRef]
- Yang, J.-C.; Dutta, P.K. Promoting selectivity and sensitivity for a high temperature YSZ-based electrochemical total NOx sensor by using a Pt-loaded zeolite Y filter. Sens. Actuators B Chem. 2007, 125, 30–39. [Google Scholar] [CrossRef]
- Yang, J.-C.; Dutta, P.K. High temperature amperometric total NOx sensors with platinum-loaded zeolite Y electrodes. Sens. Actuators B Chem. 2007, 123, 929–936. [Google Scholar] [CrossRef]
- Yang, J.-C.; Dutta, P.K. High temperature potentiometric NO2 sensor with asymmetric sensing and reference Pt electrodes. Sens. Actuators B Chem. 2010, 143, 459–463. [Google Scholar] [CrossRef]
- Buciuman, F.C.; Patcas, F.; Menezo, J.C.; Barbier, J.; Hahn, T.; Lintz, H.G. Catalytic properties of La0.8A0.2MnO3 (A = Sr, Ba, K, Cs) and LaMn0.8B0.2O3 (B = Ni, Zn, Cu) perovskites 1. Oxidation of hydrogen and propene. Appl. Catal. B Environ. 2002, 35, 175–183. [Google Scholar] [CrossRef]
- Belardi, R.M.; Deseure, J.; Brant, M.C.; Matencio, T.; Domingues, R.Z. Electrical study of cathodic activation and relaxation of La0.8Sr0.2MnO3. Ionics 2009, 15, 227–232. [Google Scholar] [CrossRef]
- Bredikhin, S.; Maeda, K.; Awano, M. Electrochemical Cell with Two Layers Cathode for NO Decomposition. Ionics 2001, 7, 109–115. [Google Scholar] [CrossRef]
- Reinhardt, G.; Wiemhofer, H.D.; Gopel, W. Electrode Reactions of La0.8Sr0.2MnO3±δ-Electrodes on Stabilized Zirconia with Oxygen and the Nitrogen Oxides NO and NO2. Ionics 1995, 1, 32–39. [Google Scholar] [CrossRef]
- Hwang, H.J.; Moon, J.W.; Awano, M. Microstructure and NO decomposition behavior of sol-gel derived (La0.8Sr0.2)0.95MnO3/yttria-stabilized zirconia nanocomposite thin film. Mater. Res. Bull. 2003, 38, 311–318. [Google Scholar] [CrossRef]
- Tietz, F.; Papadelis, C.; Tsiplakides, D.; Katsaounis, A.; Vayenas, C.G. Temperature Programmed Oxygen Desorption of the Perovskites Series Ln0.65Sr0.3Mn0.8Co0.2O3 (Ln = La-Gd). Ionics 2001, 7, 101–104. [Google Scholar] [CrossRef]
- Charpentier, P.; Fragnaud, P.; Schleich, D.M.; Denos, Y.; Gehain, E. Preparation of Thin Film SOFCs Working at Reduced Temperature. Ionics 1998, 4, 118–123. [Google Scholar] [CrossRef]
- Nagde, K.R.; Bhoga, S.S. Study of mechanochemically prepared nanocrystalline La0.8Sr0.2MnO3. Ionics 2010, 16, 361–370. [Google Scholar] [CrossRef]
- Zhang, S.; Bi, L.; Zhang, L.; Yang, C.; Wang, H.; Liu, W. Fabrication of cathode supported solid oxide fuel cell by multi-layer tape casting and co-firing method. Int. J. Hydrogen Energy 2009, 34, 7789–7794. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, R.; Wang, S.; Wen, T. Fabrication of a large area cathode-supported thin electrolyte film for solid oxide fuel cells via tape casting and co-sintering techniques. Electrochem. Commun. 2009, 11, 842–845. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, Y.; Zhou, Y.; Zhan, Z.; Wang, S. Fabrication and characterization of a cathode-support solid oxide fuel cell by tape casting and lamination. Int. J. Hydrogen Energy 2013, 38, 16584–16589. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Qian, J.; Xin, X.; Zhan, Z.; Wen, T. A novel catalytic layer material for direct dry methane solid oxide fuel cell. Int. J. Hydrogen Energy 2013, 38, 14053–14059. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Jin, H.; Zhan, Z.; Jian, J. NO2 sensing properties of electrode-supported sensor by tape casting and co-firing method. Ionics 2015. [Google Scholar] [CrossRef]
- Zhuiykov, S.; Nakano, T.; Kunimoto, A.; Yamazoe, N.; Miura, N. Potentiometric NOx sensor based on stabilized zirconia and NiCr2O4 sensing electrode operating at high temperatures. Electrochem. Commun. 2001, 3, 97–101. [Google Scholar] [CrossRef]
- Elumalai, P.; Miura, N. Performances of planar NO2 sensor using stabilized zirconia and NiO sensing electrode at high temperature. Solid State Ion. 2005, 176, 2517–2522. [Google Scholar] [CrossRef]
- Kading, S.; Jakobs, S.; Guth, U. YSZ-cells for potentiometric nitric oxide sensors. Ionics 2003, 9, 151–154. [Google Scholar] [CrossRef]
- Zhu, J.J.; Thomas, A. Perovskite-type mixed oxides as catalytic material for NO removal. Appl. Catal. B Environ. 2009, 92, 225–233. [Google Scholar] [CrossRef]
- Huang, T.J.; Wang, C.H. Effect of temperature and NOx concentration on nitric oxide removal from simulated lean-burn engine exhaust via electrochemical-catalytic cells. Chem. Eng. J. 2011, 173, 530–535. [Google Scholar] [CrossRef]
- Xiong, W.; Kale, G.M. Electrochemical NO2 sensor using a NiFe1.9Al0.1O4 oxide spinel electrode. Anal. Chem. 2007, 79, 3561–3567. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, P.; Plashnitsa, V.V.; Ueda, T.; Hasei, M.; Miura, N. Dependence of NO2 sensitivity on thickness of oxide-sensing electrodes for mixed-potential-type sensor using stabilized zirconia. Ionics 2006, 12, 331–337. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Zheng, Y.; Li, J.; Zhan, Z.; Jian, J. Potentiometric NO2 Sensors Based on Thin Stabilized Zirconia Electrolytes and Asymmetric (La0.8Sr0.2)0.95MnO3 Electrodes. Sensors 2015, 15, 17558-17571. https://0-doi-org.brum.beds.ac.uk/10.3390/s150717558
Zou J, Zheng Y, Li J, Zhan Z, Jian J. Potentiometric NO2 Sensors Based on Thin Stabilized Zirconia Electrolytes and Asymmetric (La0.8Sr0.2)0.95MnO3 Electrodes. Sensors. 2015; 15(7):17558-17571. https://0-doi-org.brum.beds.ac.uk/10.3390/s150717558
Chicago/Turabian StyleZou, Jie, Yangong Zheng, Junliang Li, Zhongliang Zhan, and Jiawen Jian. 2015. "Potentiometric NO2 Sensors Based on Thin Stabilized Zirconia Electrolytes and Asymmetric (La0.8Sr0.2)0.95MnO3 Electrodes" Sensors 15, no. 7: 17558-17571. https://0-doi-org.brum.beds.ac.uk/10.3390/s150717558